Human ovarian cancer cell inhibitor as well as preparation method and application thereof
A technology of ovarian cancer cells and inhibitors, which is applied in the field of medicine to achieve the effect of targeted inhibition of human ovarian cancer proliferation and high anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
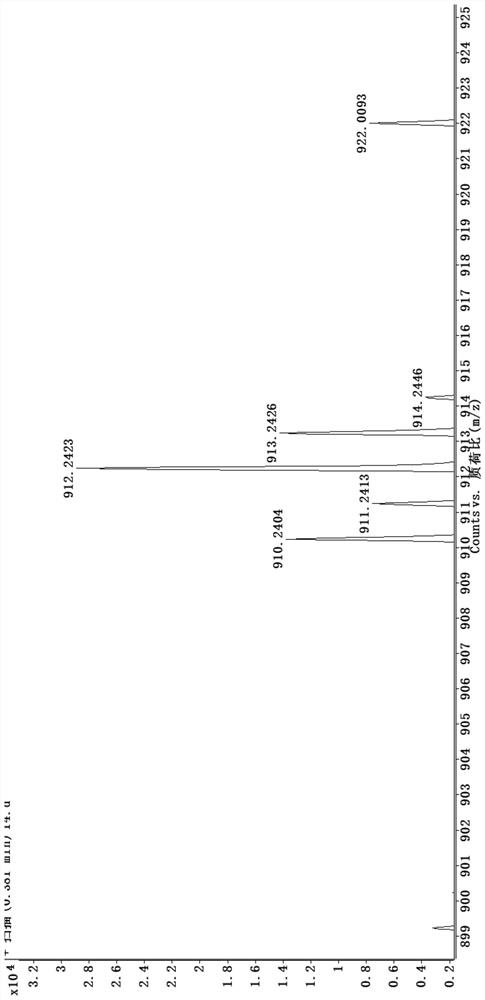
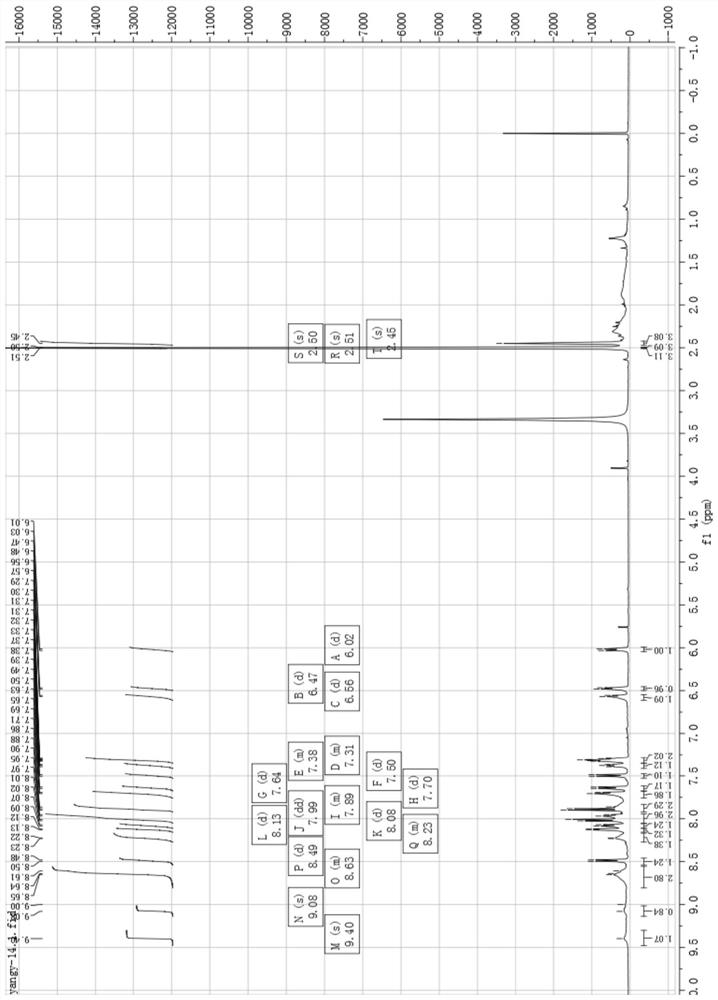
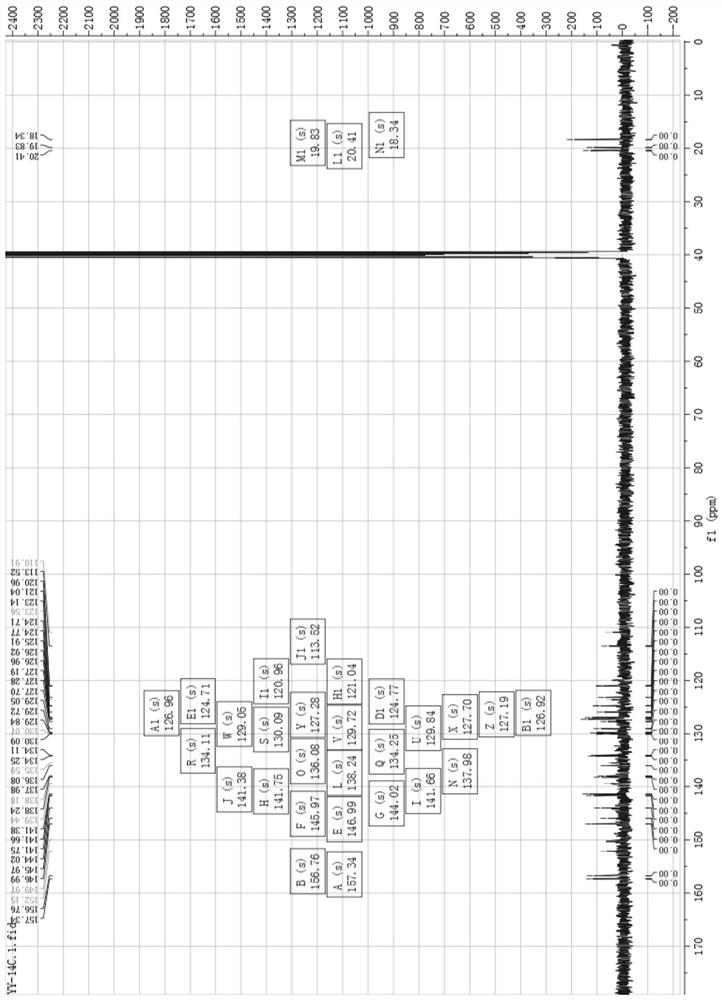
[0039] (1) Human ovarian cancer cell inhibitor [Ir(3a)(BQ) 2 ] preparation and characterization:
[0040] Such as Figure 6 As shown, weigh compound 4 and compound 3a (8-hydroxyquinoline derivative) according to the molar ratio of 1:1.3, put them into the container, then add ethylene glycol, heat and reflux for 13h under the protection of argon, and obtain a dark red clear solution ; Cool to room temperature, add water to dilute, and then add saturated ammonium hexafluorophosphate solution, which produces a large amount of red precipitate; filter with suction, wash with water and ether, and dry; dissolve the dried crude product with acetonitrile, and filter with neutral alumina Column separation; use V (dichloromethane): V (acetonitrile) = 3: 1 mixed solvent to collect the yellow component, then distill under reduced pressure and spin dry to remove the solvent to obtain a khaki solid product, which is the inhibitor of human ovarian cancer cells. Agent [Ir(3a)(BQ) 2 ], that ...
Embodiment 2
[0052] Human Ovarian Cancer Cell Inhibitor [Ir(3a)(BQ) 2 ] The synthetic route such as Figure 6 Shown:
[0053] (1) Preparation of Compound 1: Add 600mL of concentrated hydrochloric acid (pre-placed in the refrigerator to freeze, concentrated hydrochloric acid concentration is 37% mass fraction) and 14.5g of 8-hydroxyquinoline in a 1L round bottom flask, and heat to 40°C. After the 8-hydroxyquinoline is completely dissolved, add 53g NaClO in batches 3 (The addition was completed within 60 minutes), and after the addition was completed, the stirring was continued at 40° C. for 2 h. After the reaction was completed, dilute to 2L with ice water and dilute with CH 2 Cl 2 (6 × 250mL) extraction, combined organic phase, washed with 3 × 200mL of distilled water, vacuum rotary evaporation of the solvent to obtain a yellow solid, filtered the precipitate, and the solid was recrystallized three times with 40mL of methanol to obtain 6,7-dichloroquinoline-5 ,8-diketone, denoted as c...
Embodiment 3
[0059] Human Ovarian Cancer Cell Inhibitor [Ir(3a)(BQ) 2 ] The synthetic route such as Figure 6 Shown:
[0060] (1) Preparation of compound 1: carefully add 725mL of concentrated hydrochloric acid (pre-placed in the refrigerator to freeze, the concentration of concentrated hydrochloric acid is 35% mass fraction) and 14.5g of 8-hydroxyquinoline into a 1L round bottom flask, and heat to 45°C , after the 8-hydroxyquinoline is completely dissolved, add 64g NaClO in batches 3 (The addition was completed within 60 minutes), and after the addition was completed, the stirring was continued at 45° C. for 1 h. After the reaction was completed, dilute to 2L with ice water and dilute with CH 2 Cl 2 (6 × 250mL) extraction, combined organic phase, washed with 3 × 200mL of distilled water, vacuum rotary evaporation of the solvent to obtain a yellow solid, filtered the precipitate, and the solid was recrystallized three times with 40mL of methanol to obtain 6,7-dichloroquinoline-5 ,8-di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com