Glycolipid derivative based on Kdo as well as preparation and antibacterial application of glycolipid derivative
A technology of derivatives and glycolipids, which is applied in the field of synthesis of glycolipids to achieve the effects of improving the ability to penetrate the membrane and having a good inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
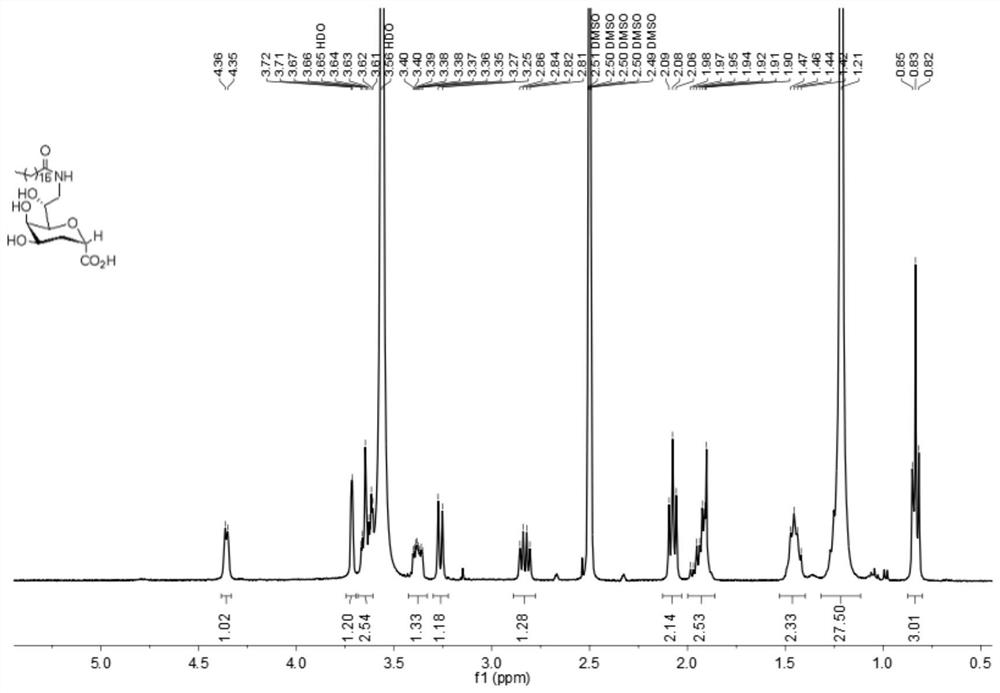
[0036] 1. Dissolve 100 mg (0.36 mmol) of compound 1 in 1 mL of methanol, add 3.6 mL of 0.5 mol / L lithium hydroxide aqueous solution dropwise to it under stirring at room temperature, and stir the resulting mixture at room temperature for 2 h, then neutralize it with an acidic ion exchange resin To neutrality, it was filtered and concentrated to obtain compound 2 (87mg, 0.36mmol, yield 100%), and the obtained crude product was directly used in subsequent reactions.
[0037]
[0038] 2. 87 mg (0.36 mmol) of compound 2 and 116 mg (0.43 mmol) of stearylamine were dissolved in 4 mL of DMF, and 89 mg (0.46 mmol) of EDCI, 63 mg (0.47 mmol) of HOBt and 108 μL ( 0.62mmol) DIPEA, stirred at room temperature for 8.5h, concentrated to remove the solvent, and the resulting syrup was dissolved in CH 2 Cl 2after dissolving with H 2 Washed with O, the organic phase was collected and washed with Na 2 SO 4 Drying, filtration and concentration, column chromatography (CH 2 Cl 2 / MeOH=10:...
Embodiment 2
[0045] 1. Dissolve 200 mg (0.73 mmol) of compound 1 in 7 mL of a mixed solution of tetrahydrofuran and deionized water at a volume ratio of 6:1, and add 526 mg (1.78 mmol) of triphenylphosphine, and heat the resulting mixture to 60°C for 7 hours; After the reaction solution was cooled to room temperature, it was concentrated to remove the solvent. The resulting syrup was dissolved in deionized water and washed three times with ethyl acetate. The aqueous phase was concentrated to obtain compound 4 as a white solid (172 mg, 0.68 mmol, yield 96%).
[0046]
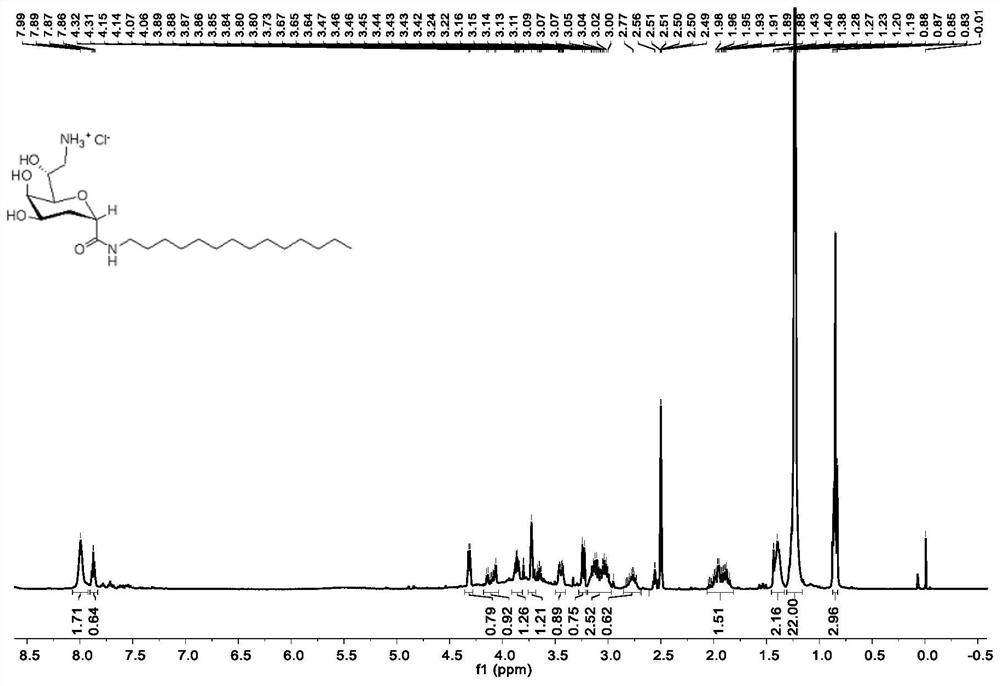
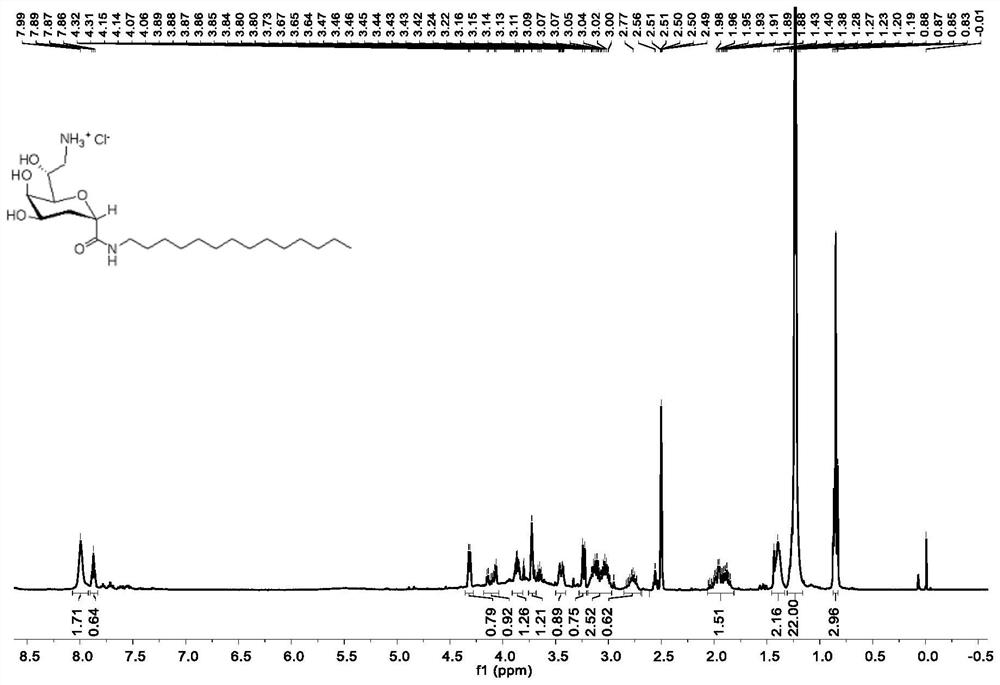
[0047] The structural characterization data of the obtained compound 4 is: 1 H NMR (400MHz, Methanol-d 4 )δ4.61–4.53(m,1H),4.30–4.18(m,2H),3.94(d,J=2.5Hz,1H),3.93–3.87(m,1H),3.64–3.54(m,1H) ,3.49(dd,J=8.5,0.8Hz,1H),3.01(dd,J=13.4,3.4Hz,1H),2.93(dd,J=13.4,5.7Hz,1H),2.20–2.09(m,2H ),1.30(t,J=7.1Hz,3H).
[0048] 2. Add 132mg (0.53mmol) of compound 4, 305mg (0.68mmol) of pentafluorophenol octadecylcarboxylate, and 111μL (0....
Embodiment 3
[0057] 1. Dissolve 396mg (1.44mmol) of compound 1 in 6mL of methanol, add 13.34mL of 0.5mol / L lithium hydroxide aqueous solution dropwise to it under stirring at room temperature, stir the resulting mixture for 4 hours at room temperature, and neutralize it with an acidic ion exchange resin To neutrality, it was filtered and concentrated to obtain compound 2 (340 mg, 1.38 mmol, yield 96%), and the obtained crude product was directly used in subsequent reactions.
[0058]
[0059] 2. Dissolve 184mg (0.73mmol) of compound 2, 173mg (0.81mmol) of saturated tetradecylamine in 4mL of DMF, and add 172mg (0.90mmol) of EDCI, 118mg (0.56mmol) of HOBt and 300μL (1.82mmol) DIPEA, stirred at room temperature for 12h, concentrated to remove the solvent, and the obtained syrup was dissolved in CH 2 Cl 2 after dissolving with H 2 Washed with O, the organic phase was collected and washed with Na 2 SO 4 Drying, filtration and concentration, column chromatography (CH 2 Cl 2 / MeOH=10:1, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com