Mogrol derivative monomer as well as preparation method and application thereof
A technology of mogrosideol and derivatives, applied in the directions of drug combination, pharmaceutical formula, steroid compound, etc., can solve the problems of lack of carboxyl compound synthesis method and anti-inflammatory effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
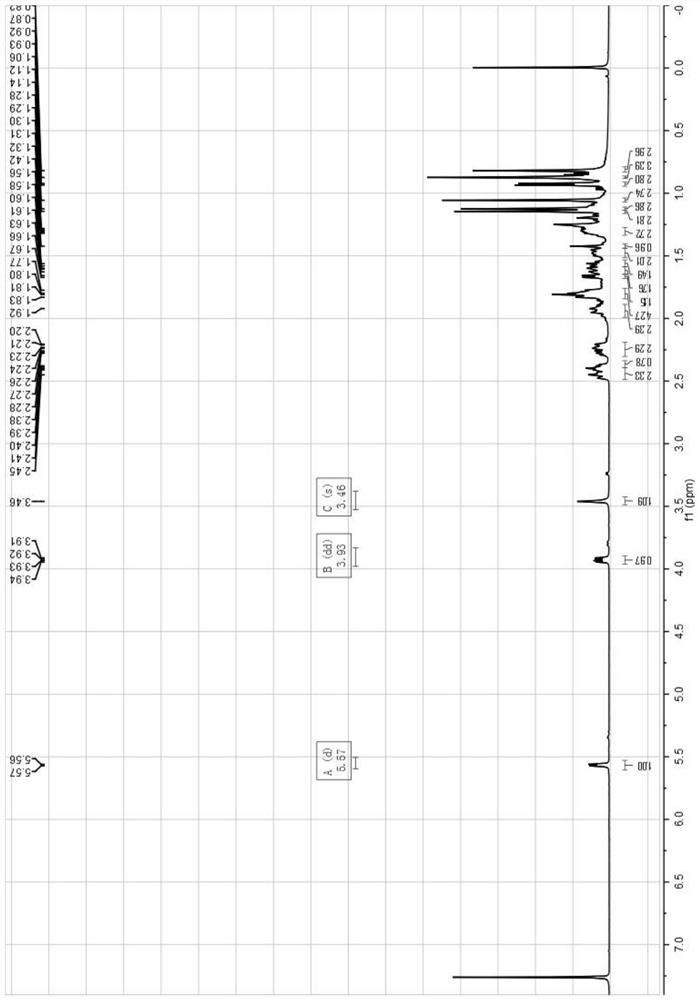
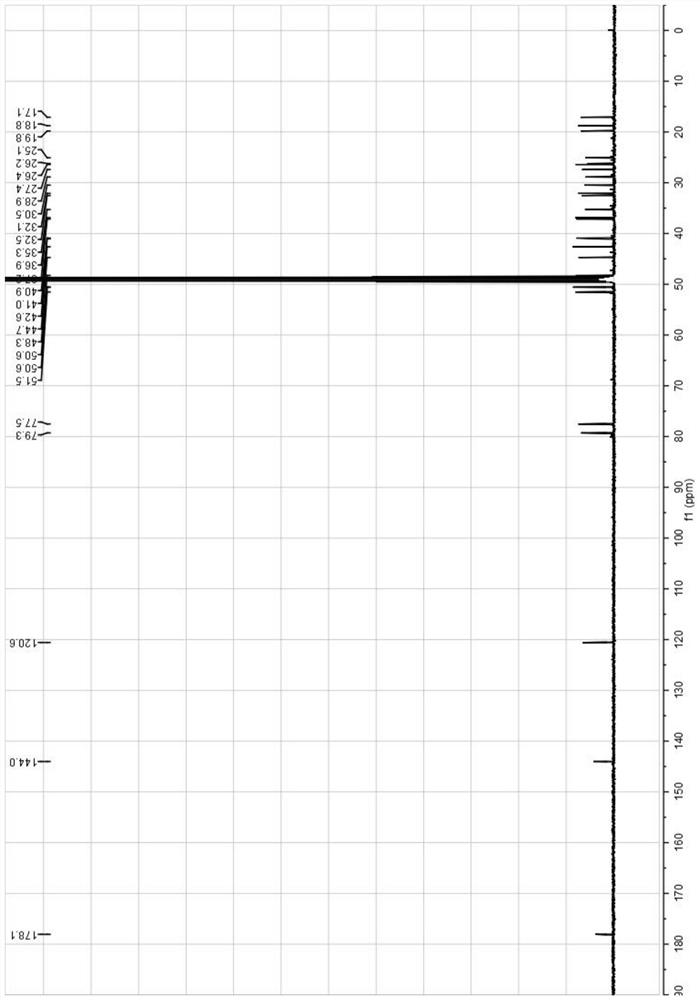
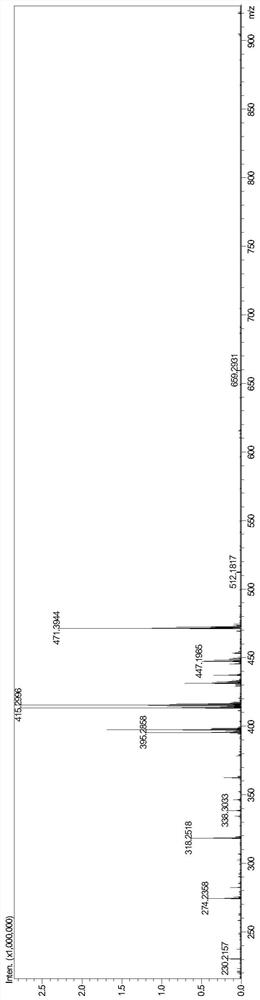
Image
Examples
Embodiment 1
[0063] The preparation method of the mogroside alcohol derivative monomer of the present embodiment comprises the following steps:
[0064] Step 1: Preparation of intermediate product M-1
[0065] Take 1.5 g of mogrosinol, dissolve it in 15 mL of acetone, add 15 mL of pure water, raise the temperature to 40°C and stir for 5 minutes to obtain mogrosinol solution.
[0066] Take 15mL of acetone, 15mL of pure water and 0.3mL of acetic acid, and mix them uniformly to obtain the mixed solution A.
[0067] Take 1.5g of sodium periodate, dissolve it in the above-mentioned mixed solution A, and mix evenly to obtain the mixed solution B.
[0068] Slowly add the above-mentioned mixture B to the above-mentioned mogrosin alcohol solution, and react in the dark at 40°C for 16 hours after the dropwise addition, and then lower it to room temperature at 20°C, remove the acetone under reduced pressure, and extract twice with ethyl acetate. is 20mL, and the second dosage is 10mL. After the org...
Embodiment 2
[0085] The preparation method of the mogroside alcohol derivative monomer of the present embodiment comprises the following steps:
[0086] Step 1: Preparation of intermediate product M-1
[0087] Take 2.5g of mogrosinol, dissolve it in 30mL of acetone, add 30mL of pure water, raise the temperature to 60°C and stir for 10min to obtain mogrosinol solution;
[0088] Take 20mL of acetone, 20mL of pure water and 0.6mL of acetic acid, and mix them uniformly to obtain the mixed solution A.
[0089] Take 2.5g of sodium periodate, dissolve it in the above-mentioned mixed solution A, and mix evenly to obtain the mixed solution B.
[0090] Slowly add the above-mentioned mixture B to the above-mentioned monk fruit alcohol solution, and react at 60°C in the dark for 24 hours after the dropwise addition, and then lower it to room temperature of 25°C, remove the acetone under reduced pressure, and extract twice with dichloromethane, the first dosage is 25mL, and the second dosage is 10mL....
Embodiment 3
[0100] The preparation method of the mogroside alcohol derivative monomer of the present embodiment comprises the following steps:
[0101] Step 1: Preparation of intermediate product M-1
[0102] Take 3.0 g of mogrosinol, dissolve it in 50 mL of acetone, add 50 mL of pure water, raise the temperature to 60°C and stir for 15 minutes to obtain mogrosinol solution.
[0103] Take 30mL of acetone, 30mL of pure water and 1.0mL of acetic acid, and mix them uniformly to obtain the mixed solution A.
[0104] Take 3.0 g of sodium periodate, dissolve it in the above-mentioned mixed solution A, and mix it uniformly to obtain the mixed solution B.
[0105] Slowly add the above-mentioned mixture B to the above-mentioned monk fruit alcohol solution, and react at 60°C in the dark for 30 hours after the dropwise addition, and then lower it to room temperature of 30°C, remove the acetone under reduced pressure, and extract twice with chloroform, the amount used for the first time is 20mL, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Separation yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com