Pharmaceutical composition and preparation method thereof
A composition and drug technology, applied in the field of medicine, can solve the problems of control, complicated preparation process, and undisclosed specific surface area of magnesium stearate, etc., and achieve the effect of improving stability, simple process, and good uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1: Comparing the impact of different bulk drug particle sizes on various properties of the product
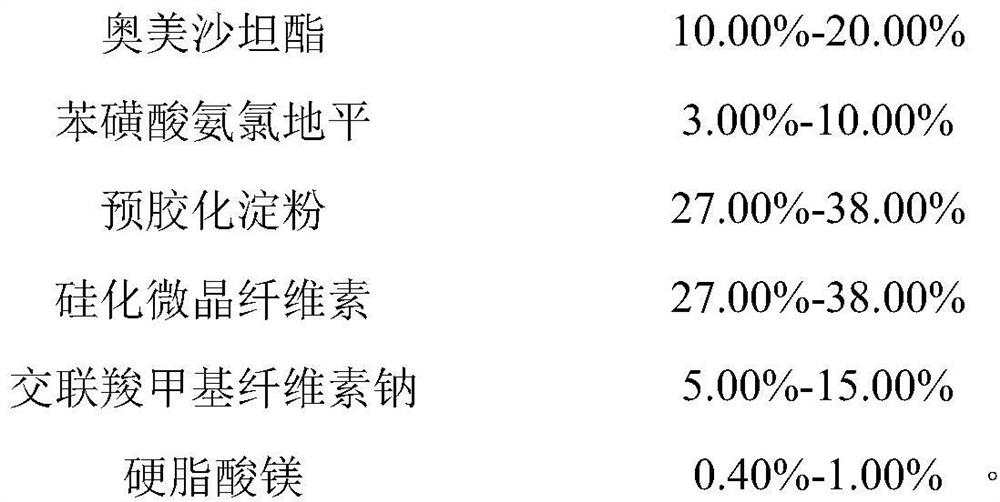
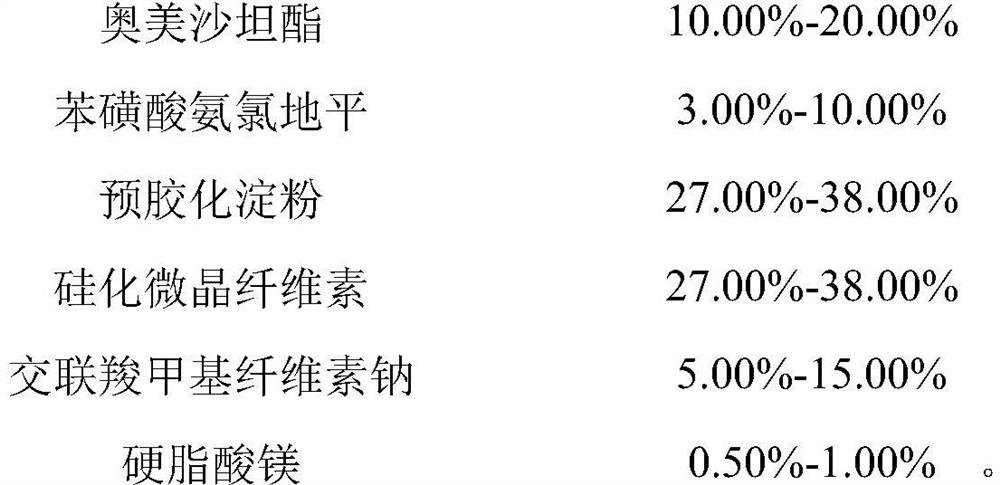
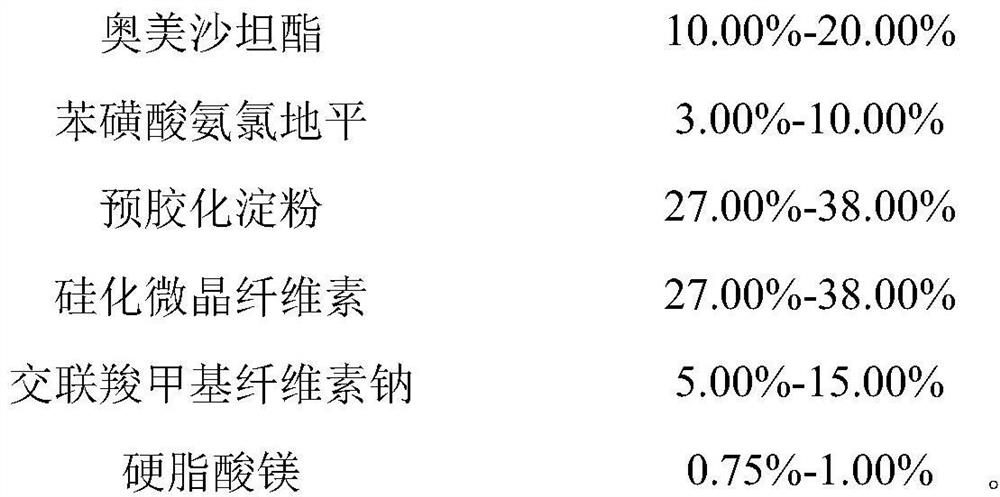
[0079] Prepare the prescription composition of 1000 tablets (specification 20mg / 5mg)
[0080]
[0081]
[0082] Wherein the specific surface area of the magnesium stearate is 5.6m 2 / g.
[0083] Preparation: mechanically pulverize olmesartan medoxomil and amlodipine besylate to the target particle size range, then pregelatinized starch, olmesartan medoxomil, amlodipine besylate, silicified microcrystalline cellulose and Croscarmellose sodium is mixed, then passed through a 20-mesh sieve, and then the prescription amount of magnesium stearate is added for total blending. After the mixing is finished, perform direct tablet compression, the main compression pressure is 8.0-20.0kN, and the tablet hardness is 30N-75N (punching: a flat hole with a diameter of 6.0mm). Prepare the coating solution, add purified water into the mixing tank, turn on the stirri...
Embodiment 2
[0103] Embodiment 2. investigate the impact of the magnesium stearate of different contents on industrialized scale-up production
[0104] The specific surface area and feed ratio of magnesium stearate in the small test scale and the enlarged batch were studied respectively, so as to ensure that the dissolution of the final product was qualified on the basis of ensuring no sticking and punching.
[0105] Table 5: The amount and specific surface area of different magnesium stearate investigated in the small test scale (batch 2-1~2-5)
[0106]
[0107] Table 6: Investigate the amount of magnesium stearate and the specific surface area of the enlarged batch of products (batch 2-6~2-9)
[0108]
[0109] The preparation method was prepared according to the preparation method disclosed in Example 1, wherein the particle size d(0.9) of olmesartan medoxomil was 23.101 μm, and the particle size d(0.9) of amlodipine was 60.321 μm.
[0110] Prepare each batch of products accor...
Embodiment 3
[0117] Example 3. Tablets made by batches 2-6, 2-7 are compared with commercially available formulations
[0118] Commercially available preparation 1 (manufacturer: Daiichi Sankyo Pharmaceutical (Shanghai) Co., Ltd., product produced by the original manufacturer, trade name: ), commercially available preparation 2 (manufacturer: Daiichi Sankyo Europe GmbH, the product produced by the original manufacturer in Germany, trade name: ) self-made preparations (batches 2-6, 2-7) to carry out the accelerated test, the accelerated test conditions are 40 ℃ ± 2 ℃, 75% RH ± 5% RH, measured the self-made tablets and commercially available preparations respectively after 6 months Related substances and dissolution rates.
[0119] Table 8: Comparison of quality between self-made batches 2-6, 2-7 samples and commercially available preparations
[0120]
[0121] It can be seen from the data in the above table that the level of related substances in the tablet prepared according to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com