Synthetic method of arecoline
A synthetic method and the technology of arecoline, which are applied in the field of synthesis of arecoline, can solve the problems of low single-step yield, high toxicity of methyl iodide, and high toxicity of dimethyl sulfate, and achieve high yield, simple preparation method, and high-quality products. The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
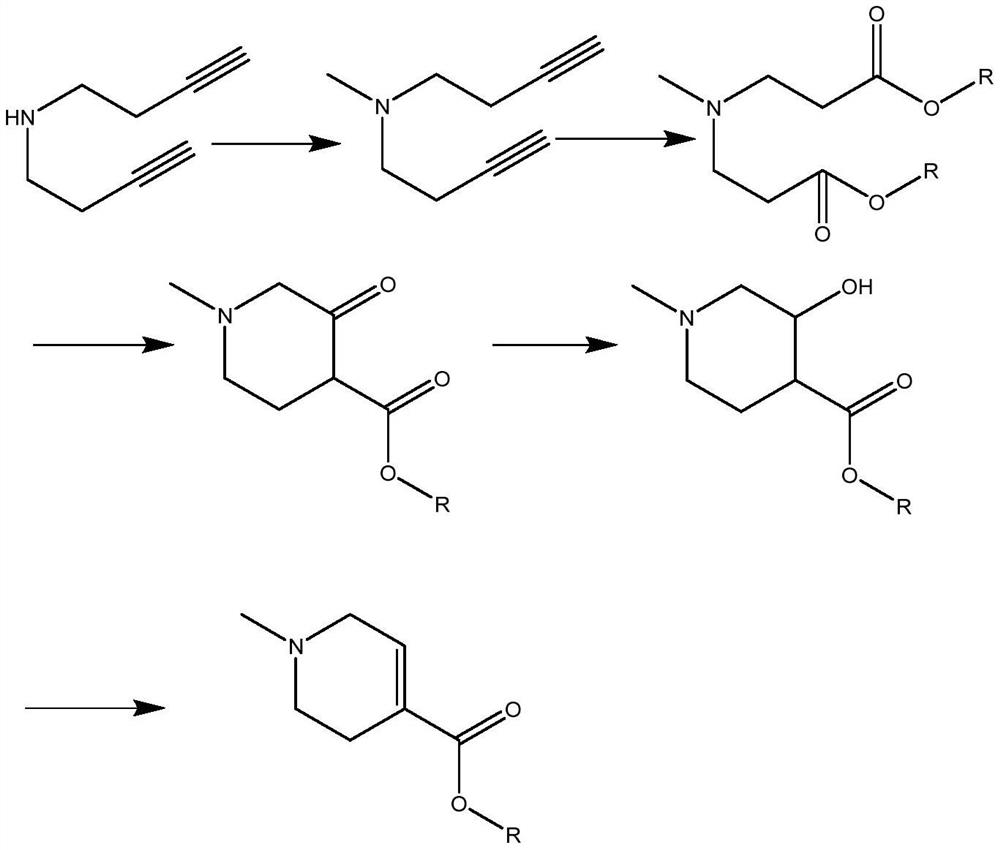
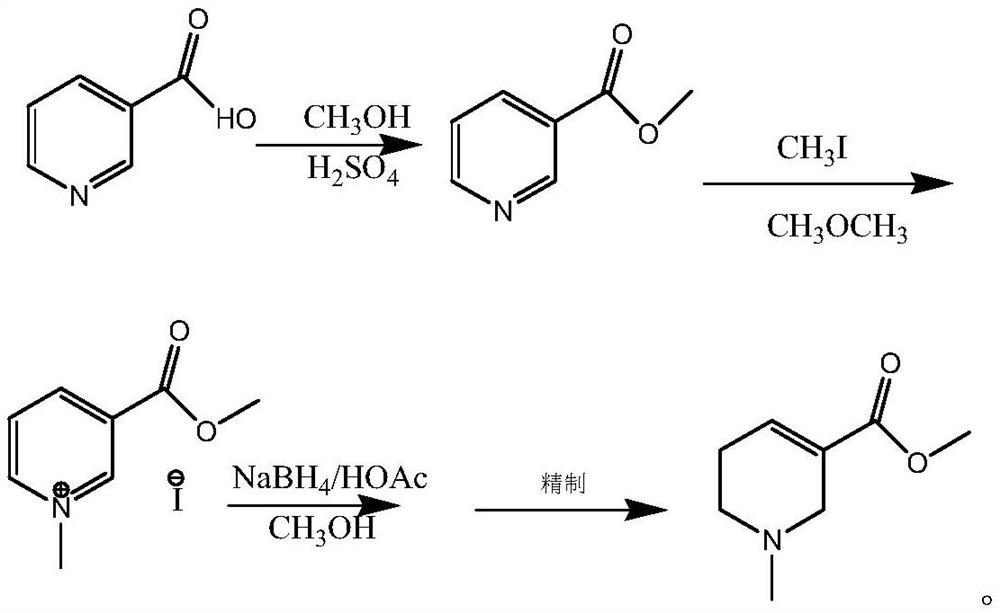
Image
Examples
Embodiment 1
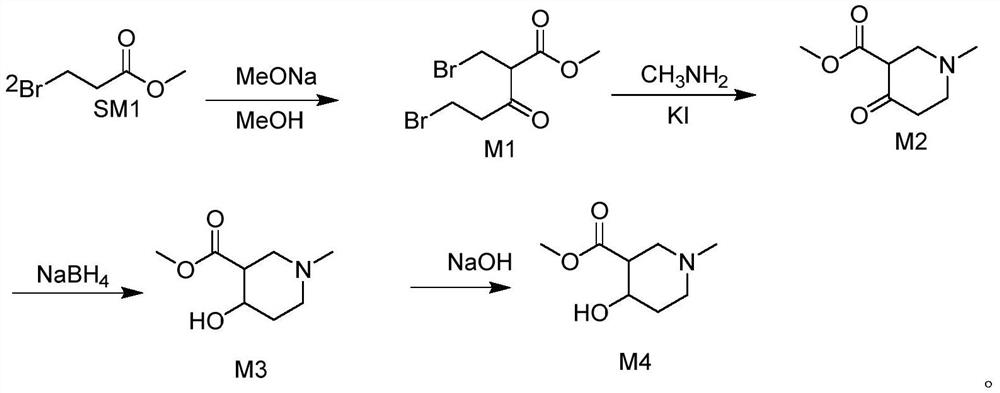
[0033] 1) Synthesis of M1
[0034]
[0035] Add 50g (0.299mol) methyl 3-bromopropionate, 54g methanol solution of sodium methoxide (30wt%) and 150ml methanol to the reaction flask, heat to reflux for 5 hours, cool down to 20-30°C after reaction, add 15g formic acid dropwise After 1.5 hours, the dropwise addition was completed, and after stirring for half an hour, suction filtration (precipitated impurities, such as sodium formate), washing the filter cake, obtained a methanol solution of M1.
[0036] 2) Synthesis of M2
[0037]
[0038] Add M1 methanol solution, 28g methylamine methanol solution (30wt%) and 0.4g KI into the reaction flask, and react at 35-45°C for 7.5h. After the reaction, cool down to 10-20°C, add 15ml of water, extract with dichloromethane, wash with 15ml of 10% dilute hydrochloric acid, wash with 15ml of saturated saline, and distill the dichloromethane to dryness under reduced pressure to obtain 38.4g of compound M2. Yield: 75.01% Purity: 98.22%. ...
Embodiment 2
[0046] 1) Synthesis of M1
[0047] Add 50g (0.299mol) methyl 3-bromopropionate, 74g sodium methoxide methanol solution (30wt%), and 150ml methanol to the reaction flask, and heat to reflux for 6 hours. After 1 hour of dropwise addition, after stirring for half an hour, suction filtration was performed, and the filter cake was washed to obtain a methanol solution of M1.
[0048] 2) Synthesis of M2
[0049] Add M1 methanol solution, 28g methylamine methanol solution (30wt%) into the reaction flask, add 0.4g KI, and react at 35-45°C for 7h. Cool down to 10-20°C after the reaction, add 15ml of quantitative water, extract with dichloromethane, wash with 15ml of 10% dilute hydrochloric acid solution, wash with saturated brine, distill dichloromethane to dryness, and obtain 38.7g of compound M2, yield: 75.59%; purity: 98.16%.
[0050] 3) Synthesis of M3
[0051] Add 35g (0.204mol) of compound M2, 150ml of methanol, and 11.7g of sodium borohydride into the reaction flask for 10 ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com