Glucosamine chondroitin tablet containing active peptide and organic calcium and preparation method of glucosamine chondroitin tablet
A technology of plain tablets and active peptides, which is applied in the field of glucosamine chondroitin tablets and its preparation, can solve the problems of tablet mold damage, unfavorable large-scale production, and low absorption and utilization rate of calcium ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 30
[0051] In the following examples 1-30, a glucosamine chondroitin tablet containing active peptide and organic calcium and its preparation method are provided. in,
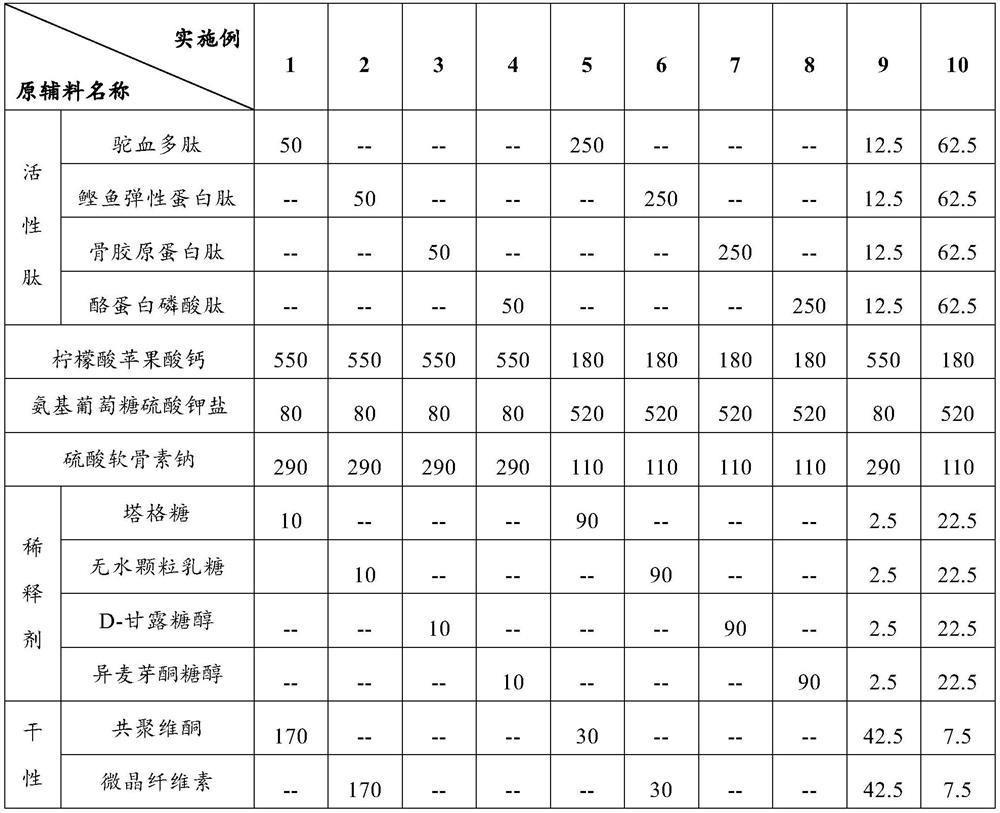
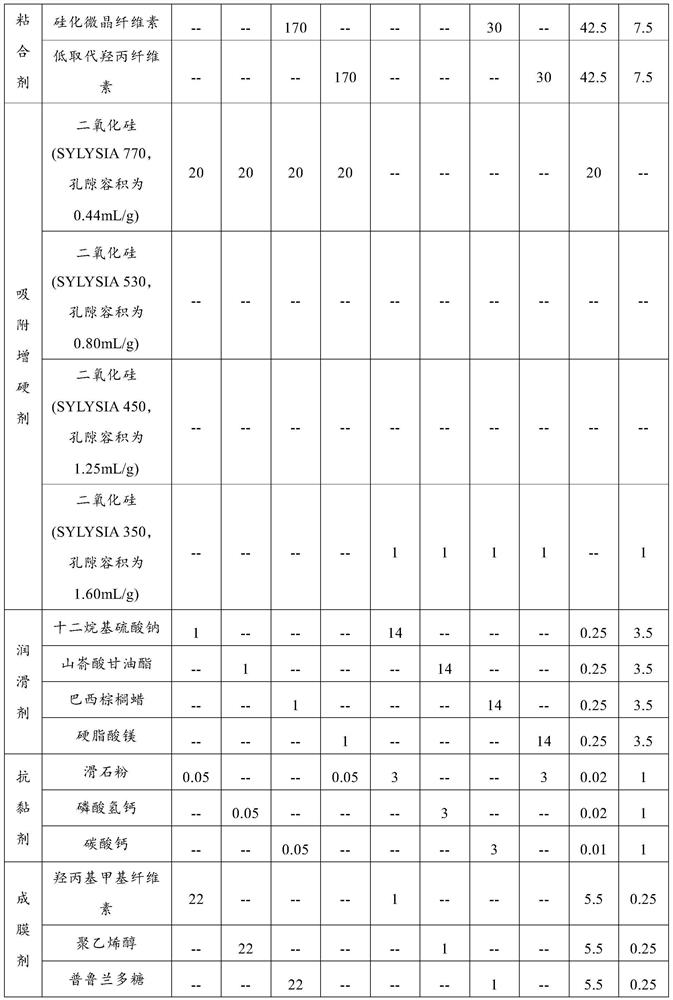
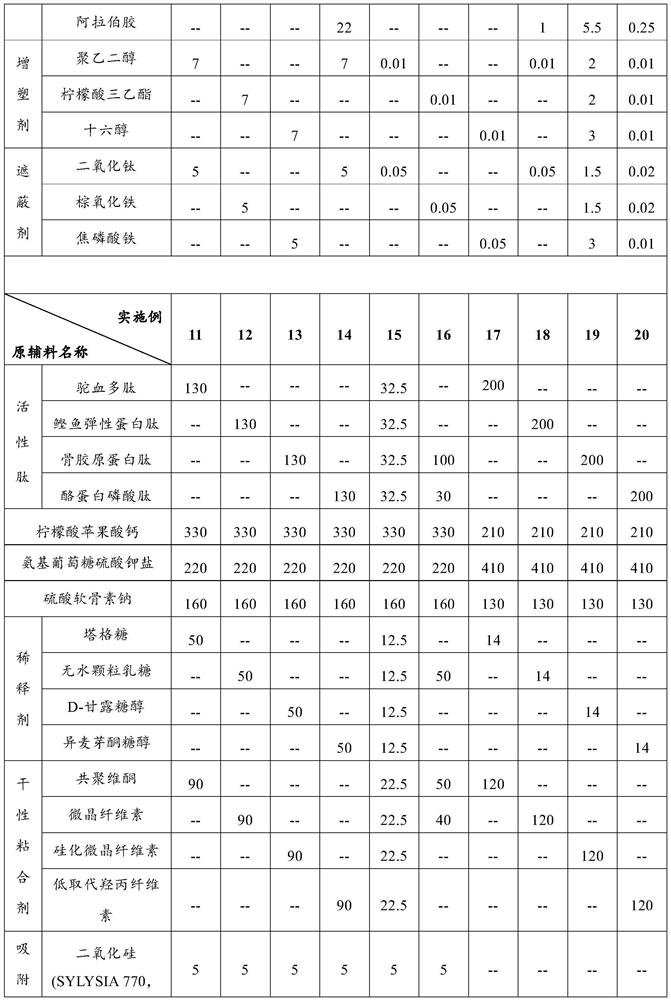
[0052] Formula: the formula composition of the glucosamine chondroitin tablet containing active peptide and organic calcium is shown in Table 1.
[0053] Preparation method: Prepare the corresponding glucosamine chondroitin tablets containing active peptide and organic calcium according to the following method:
[0054] (1) Pass the active peptide, calcium citrate malate, diluent and dry adhesive through a 60-mesh sieve respectively, pass chondroitin sulfate sodium and lubricant through a 80-mesh sieve respectively, and pass the anti-sticking agent, film-forming agent, The plasticizer and masking agent are respectively passed through a 100-mesh sieve for subsequent use;
[0055] (2) Mix glucosamine sulfate potassium salt and adsorption hardening agent for 5 minutes at a rotating speed of 10 r / min to obtain materi...
Embodiment 31
[0071] Prescription: form with embodiment 16 prescription.
[0072] Preparation:
[0073] (1) Pass the active peptide, calcium citrate malate, sodium chondroitin sulfate, diluent, dry adhesive, and lubricant through a 40-mesh sieve respectively; Pass through 80-mesh sieve respectively, set aside;
[0074] (2) Mix glucosamine sulfate potassium salt and adsorption hardening agent for 2 minutes at a rotating speed of 10 r / min to obtain material I;
[0075] (3) Pass material I through a 50-mesh sieve, and mix for 3 minutes at a rotating speed of 20 r / min to obtain material II;
[0076] (4) Mix the sieved active peptide, calcium citrate malate, sodium chondroitin sulfate, diluent, dry adhesive, lubricant and material II at a speed of 5 r / min for 45 minutes to obtain the material III;
[0077] (5) The tablet hardness is controlled to be 80N, and the material III is pressed into tablets to obtain the material IV;
[0078] (6) Mix the anti-adhesive agent, film-forming agent, plas...
Embodiment 32
[0082] Prescription: form with embodiment 30 prescription.
[0083] Preparation method: (1) Pass the active peptide, calcium citrate malate, sodium chondroitin sulfate, diluent, dry adhesive and lubricant through an 80-mesh sieve respectively; and masking agent are respectively passed through a 400-mesh sieve for subsequent use;
[0084] (2) Mix glucosamine sulfate potassium salt and adsorption hardening agent for 8 minutes at a rotating speed of 20r / min to obtain material I;
[0085] (3) Pass material I through a 80-mesh sieve, and mix for 10 minutes at a rotating speed of 10 r / min to obtain material II;
[0086] (4) Mix the sieved active peptide, calcium citrate malate, sodium chondroitin sulfate, diluent, dry adhesive, lubricant and material II at a speed of 20r / min for 15 minutes to obtain the material III;
[0087] (5) The tablet hardness is controlled to be 250N, and material III is pressed into tablets to obtain material IV;
[0088] (6) Mix the anti-adhesive agent, f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore volume | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com