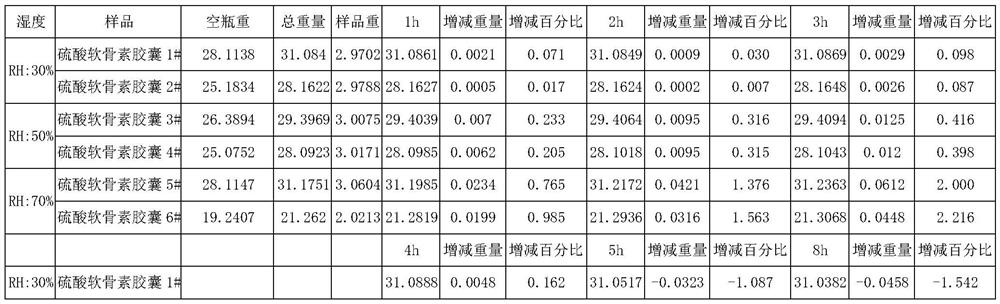
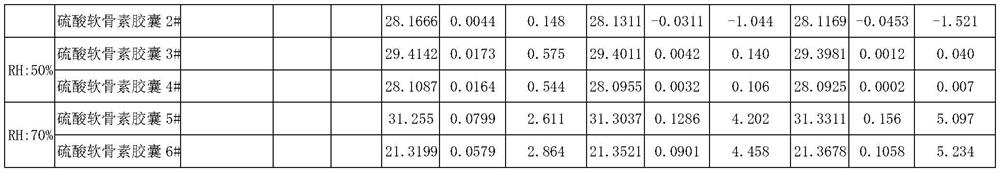
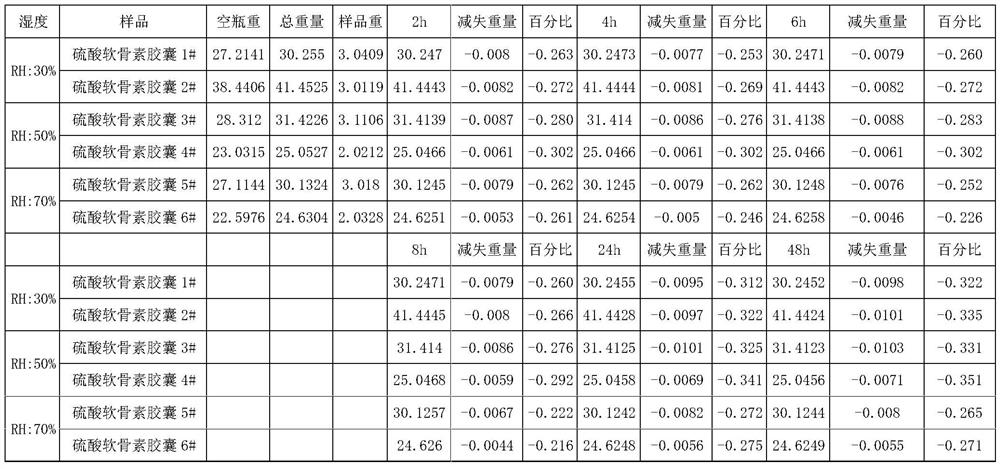
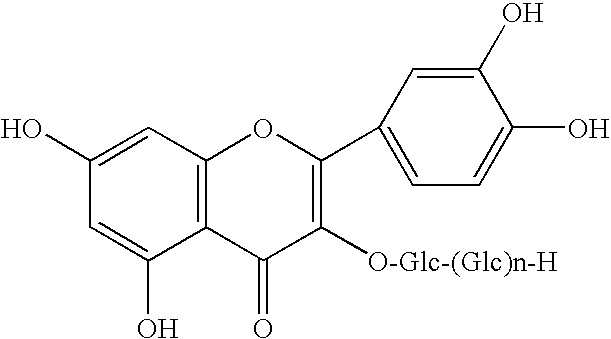
Patents
Literature
37 results about "Chondroitin sulfate sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chondroitin Sulfate Sodium. Chondroitin, hydrogen sulfate, sodium salt [9082-07-9]. » Chondroitin Sulfate Sodium is the sodium salt of the sulfated linear glycosaminoglycan obtained from bovine, porcine, or avian cartilages of healthy and domestic animals used for food by humans.
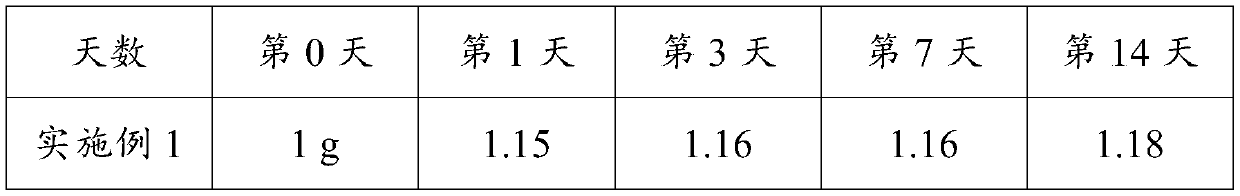
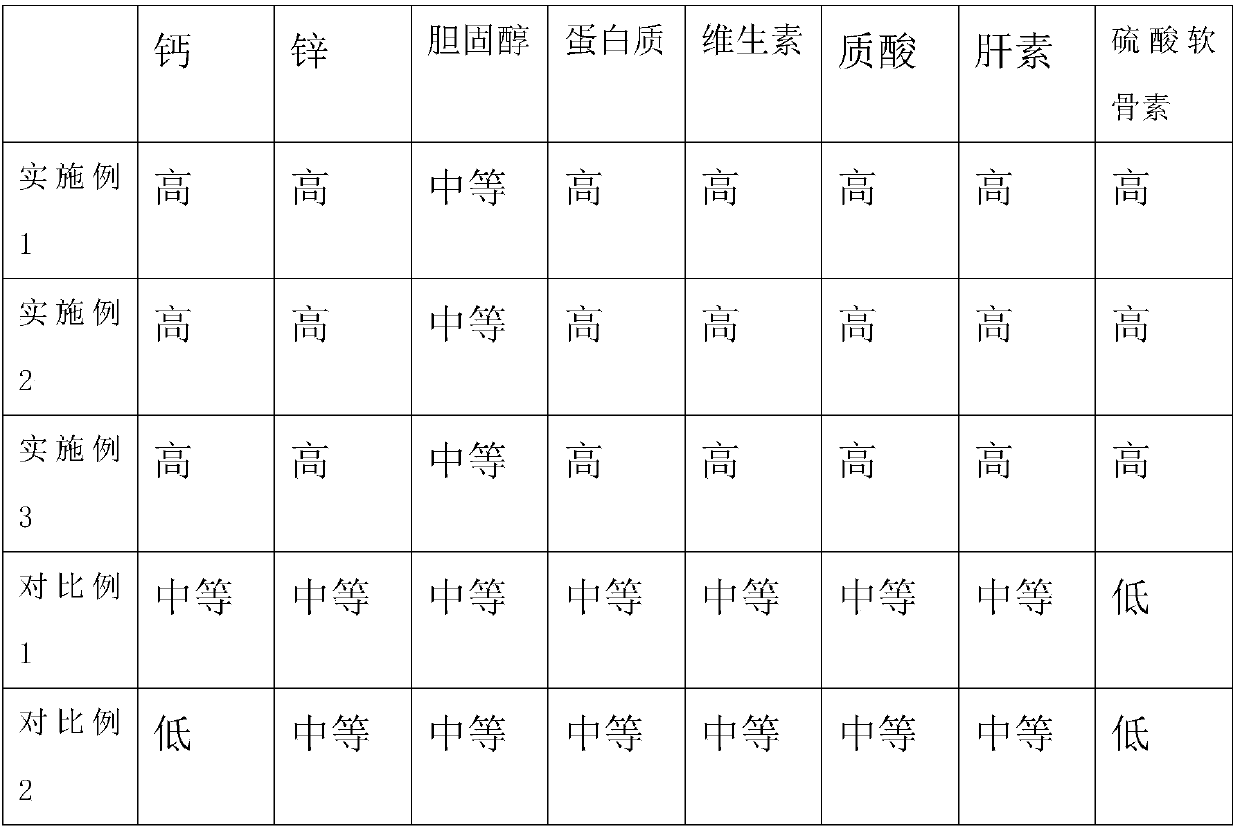
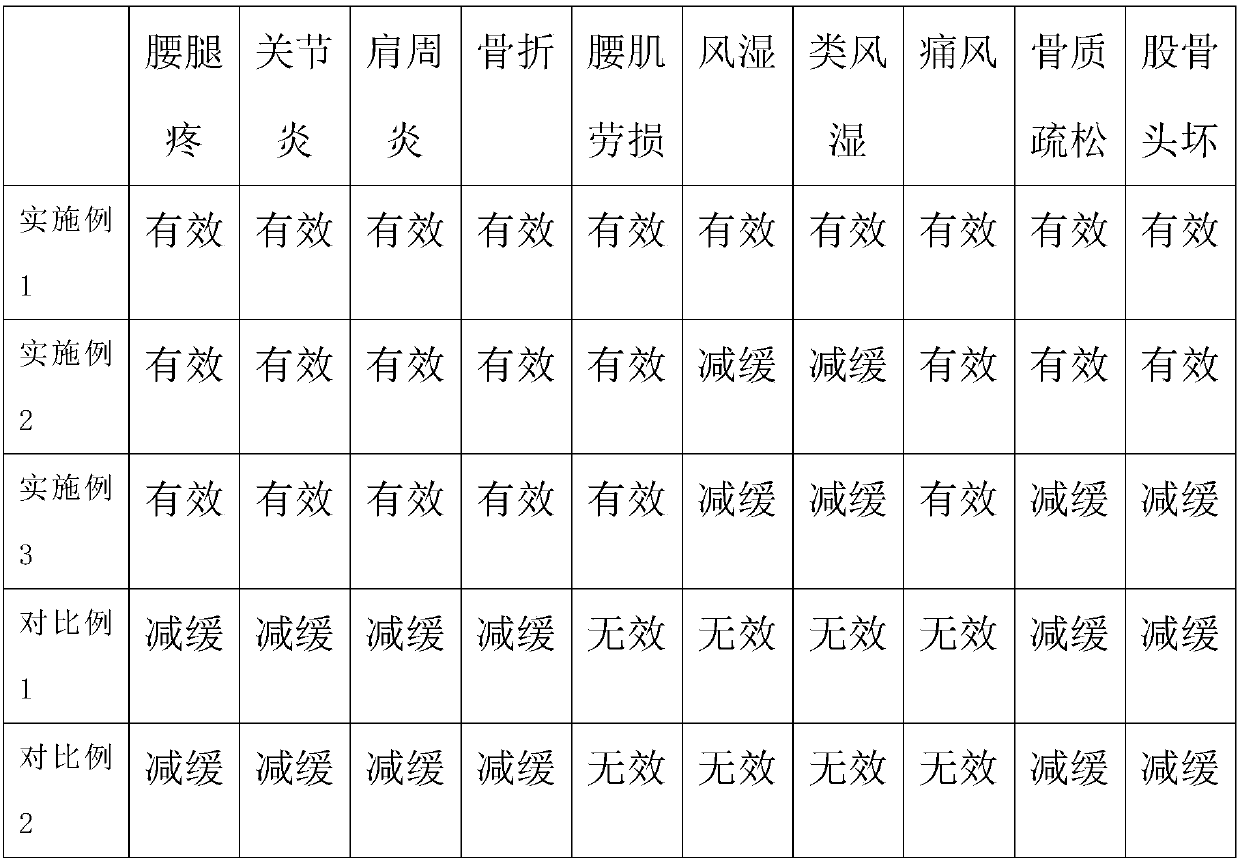
Medicine composition for treating osteoporosis and preparation method thereof
ActiveCN104306959AImprove securityPromote healingOrganic active ingredientsPeptide/protein ingredientsBone densityAdditive ingredient
The invention discloses a medicine composition for treating osteoporosis and a preparation method thereof. The medicine composition is prepared from collagen, chondroitin sulfate sodium salt, calcium carbonate, rhizoma drynariae extract, kudzu vine root extract, D-glucosamine hydrochloride and pseudo-ginseng. The medicine composition can be used for treating the pathogenesis, namely destruction of bone microstructure, of osteoporosis, is capable of solving the problem of bone density abnormality and radically preventing and treating osteoporosis; and the medicine composition can be used for relieving symptoms of ostealgia of osteoporosis patients, healing of bone fracture can be promoted, and the living quality of osteoporosis patients can be improved.
Owner:HEBEI YUZHILIN PHARMA
Enzymatic method for preparing chondroitin sulfate
The invention discloses an enzymatic method for preparing chondroitin sulfate and belongs to the medical field. Chondroitin is used as a substrate, chondroitin sulfate A with bioactivity is formed through catalysis of chondroitin 4-sulfate transferase for microbial cell heterologous expression or chondroitin sulfate C with bioactivity is formed through catalysis of chondroitin 6-sulfate transferase, and a PAPS sulfuric donor and a regeneration system are provided by acyl sulfate transferase. A new way is provided for synthesis and industrial production of biological active substances, namely, chondroitin sulfate A and chondroitin sulfate C.
Owner:JIANGNAN UNIV
Process for improving quality and yield of collagen peptide coproduced from sodium chondroitin sulfate
PendingCN111793145AHigh extraction process requirementsReduce dosageConnective tissue peptidesPeptide preparation methodsUltrafiltrationIon exchange
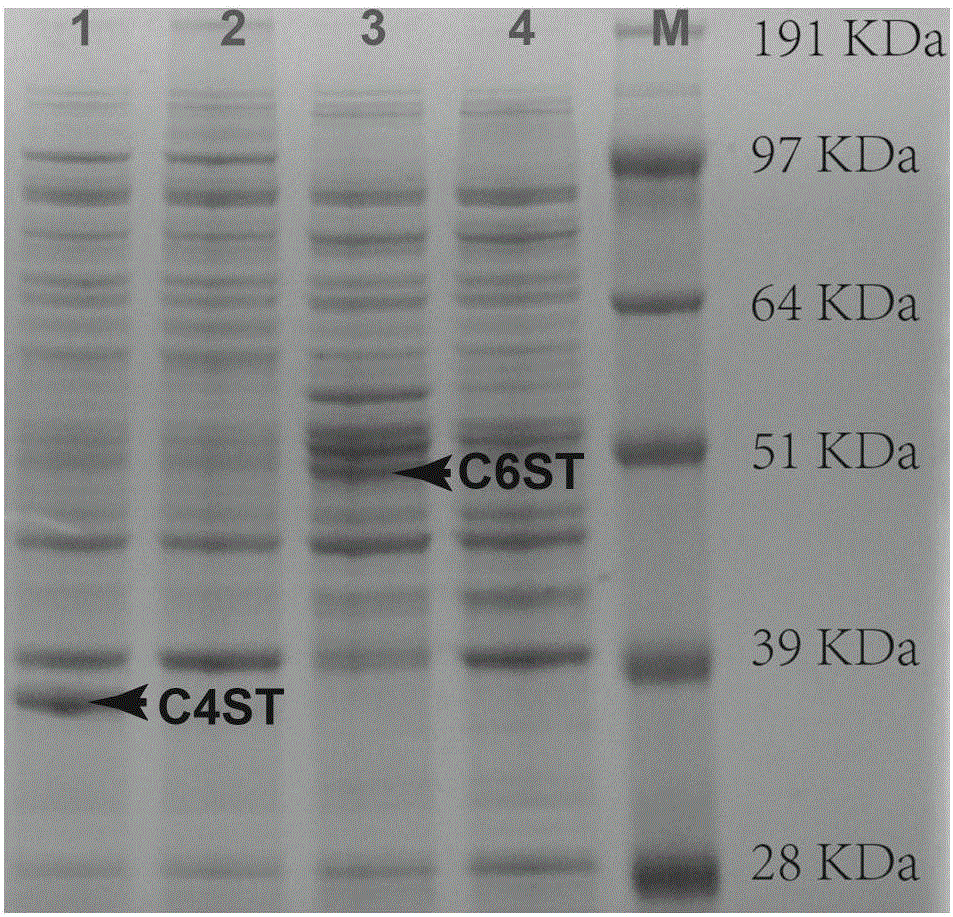
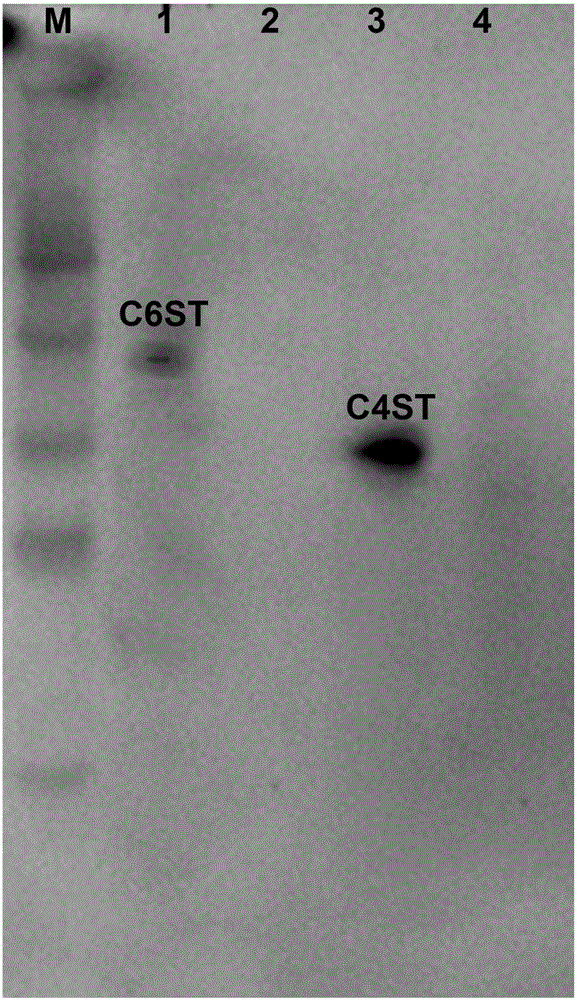
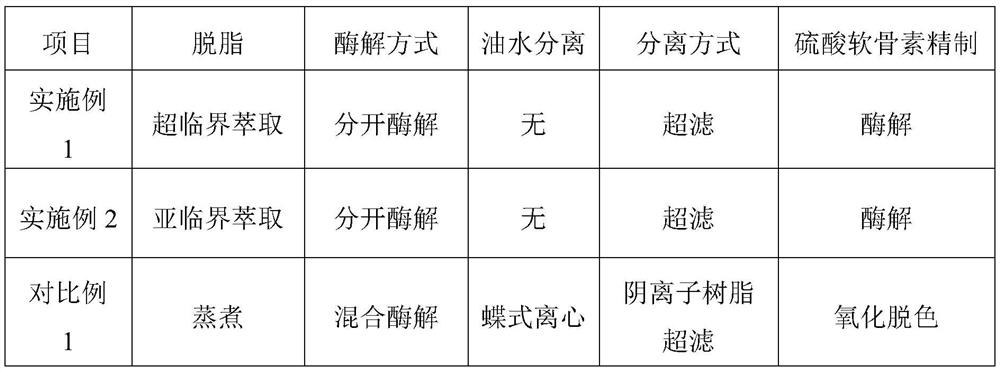
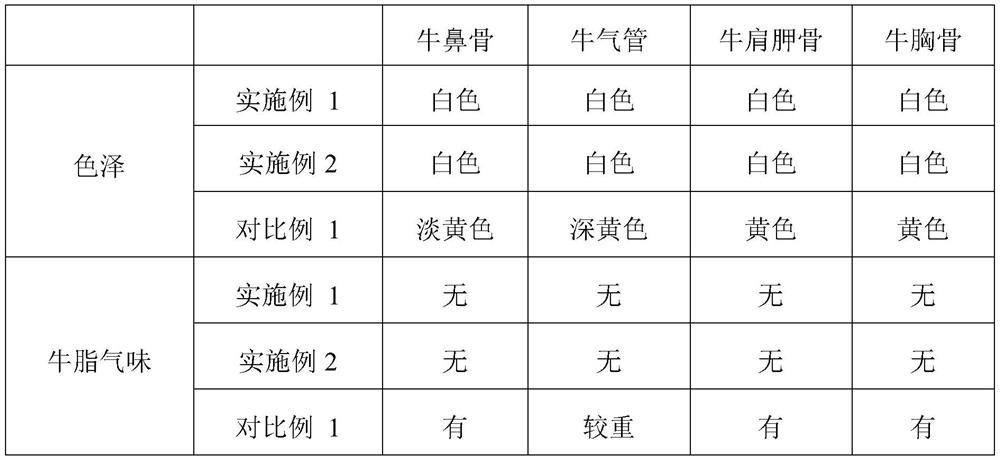
A process for improving quality and yield of collagen peptide coproduced from sodium chondroitin sulfate comprises the following steps: (1) degreasing bovine cartilage by supercritical / subcritical extraction; (2) hydrolyzing, flocculating and separating endonuclease; (3) carrying out ultrafiltration to separate chondroitin sulfate sodium and collagen peptide; (4) refining the sodium chondroitin sulfate; and (5) refining the collagen peptide. The process is used for extracting the dried bovine cartilage chondroitin sulfate sodium and collagen peptide, meanwhile, the product yield and quality are improved, and the obtained product is white in color and luster and free of beef tallow smell. According to the process, the dried bovine bones are adopted, so that the problems that domestic bovinefeeding and slaughtering are scattered, and fresh bovine cartilage is high in storage, collection and transportation cost and high in difficulty are solved; and the process is simple in technologicalprocess, easy to filter, short in production time, capable of saving energy and reducing emission, low in production cost and remarkable in economic benefit and social benefit, and processes such asresin decoloration and ion exchange are not needed.
Owner:HUNAN WUXING BIOLOGICAL TECH CO LTD
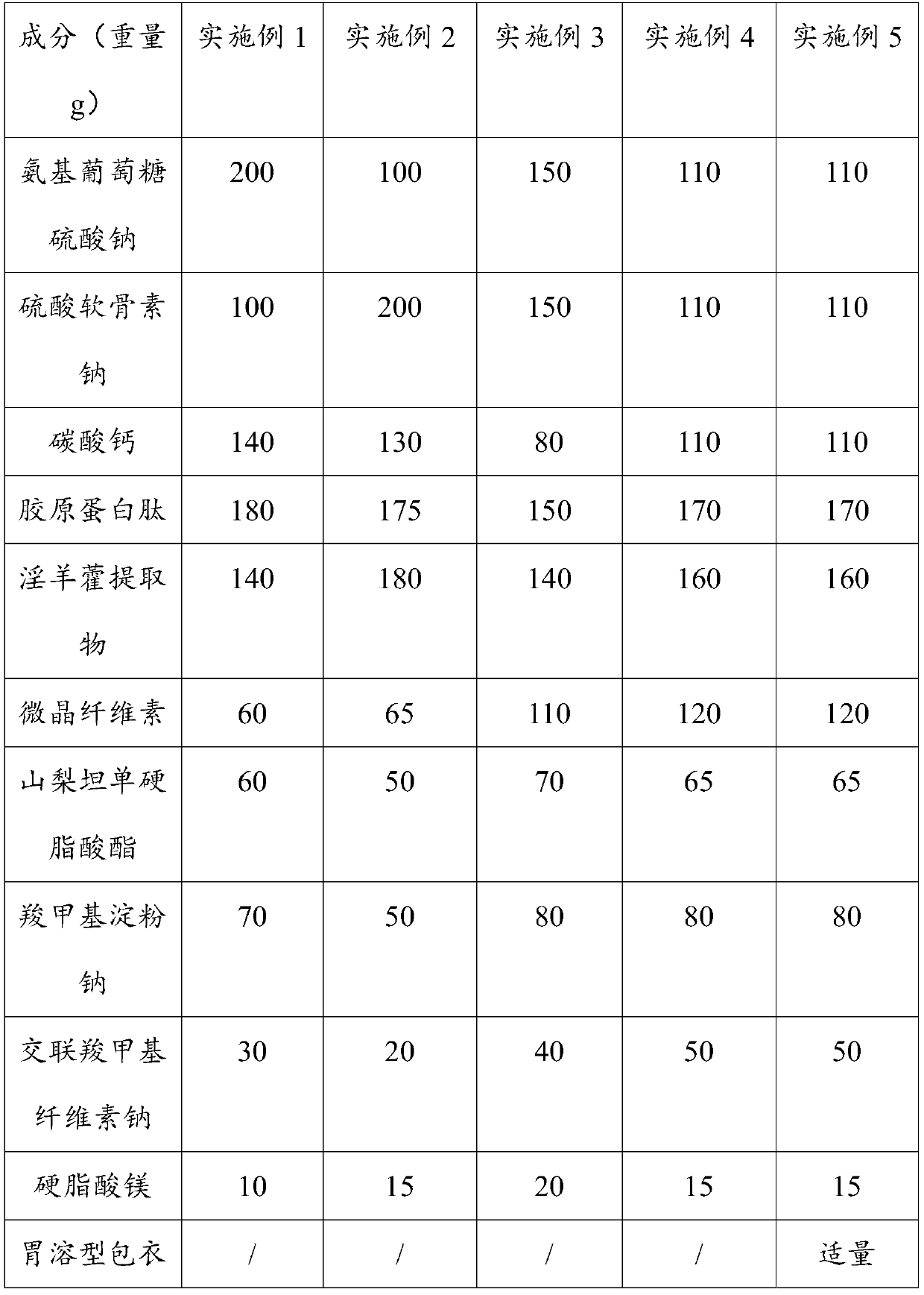
Glucosamine chondroitin calcium tablet and preparation method thereof
InactiveCN111000817AImprovement effectIncrease health functionOrganic active ingredientsPeptide/protein ingredientsBiotechnologyStearic acid
Relating to the technical field of health food, the invention provides a glucosamine chondroitin calcium tablet and a preparation method thereof. The glucosamine chondroitin calcium tablet disclosed by the invention is composed of the following raw materials: 100-200 weight parts of glucosamine sodium sulfate, 100-200 weight parts of sodium chondroitin sulfate, 80-140 weight parts of calcium carbonate, 150-180 weight parts of collagen peptide, 140-180 weight parts of a herba epimedii extract and 200-350 weight parts of an auxiliary material. The cooperative use of sorbitan monostearate and microcrystalline cellulose improves the cohesion among particles, and enhances the hardness of the glucosamine chondroitin calcium tablet, so that the prepared glucosamine chondroitin calcium tablet doesnot easily absorb external moisture, is not easily damped and is not oxidized easily, therefore the improving effect of the calcium tablet on osteoarthritis is stable.
Owner:众生健康(广东)科技有限公司
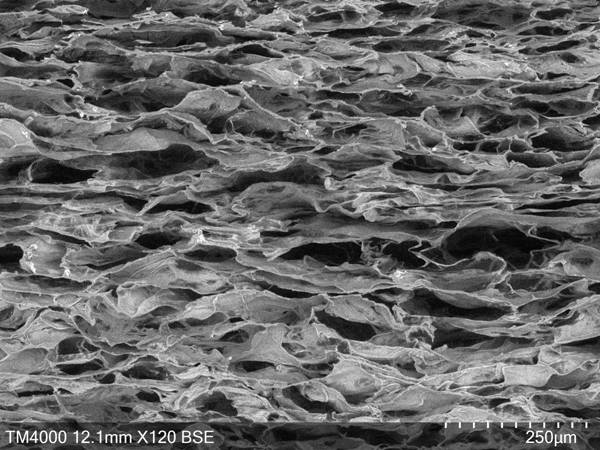
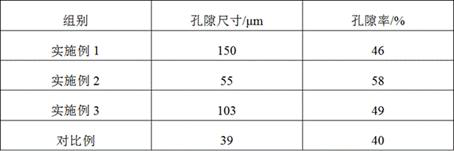
Tissue-guided regenerated collagen membrane and preparation method thereof
The invention relates to a tissue-guided regenerated collagen membrane and a preparation method thereof. The preparation method comprises the following steps: swelling type I collagen in an acetic acid solution, adding chondroitin sulfate sodium, and uniformly stirring to obtain collagen-chondroitin sulfate sodium slurry; drying the collagen-chondroitin sulfate sodium slurry to obtain a dried membrane; mixing the dried diaphragm with a crosslinking solution, and carrying out a crosslinking reaction to obtain a crosslinked collagen diaphragm after the crosslinking reaction is completed; and cleaning the cross-linked collagen membrane, carrying out secondary drying, and performing pressing after the secondary drying is completed. The preparation method comprises the following steps: firstly, swelling high-concentration I-type collagen in an acetic acid solution, adding sodium chondroitin sulfate, stirring, and then drying, cross-linking and secondarily drying, so that the obtained tissue-guided regenerated collagen membrane has excellent mechanical properties, and the pore size and porosity are beneficial to cell growth and repair; in addition, the degradation period is long, and sensitization is not prone to occurring; and the comprehensive effect is comprehensive, and the applicability is wide.
Owner:TIANXINFU (BEIJING) MEDICAL APPLIANCE CO LTD
Method for reducing ethanol residues in sodium chondroitin sulfate
The invention relates to a method for reducing ethanol residues in sodium chondroitin sulfate. Sodium chondroitin sulfate is a mucopolysaccharide substance extracted from animal cartilage such as pig nasal bone and the like. Ethanol is mainly used for precipitation purification and dehydration. According to the method, the content of ethanol in chondroitin sulfate is decreased through thermal precipitation and dehydration of ethanol, and ethanol residues are reduced. The method adopts a simple process and mild conditions, production is safer, the ethanol residue content is low, the yield of chondroitin sulfate can be increased, and the purity of chondroitin sulfate can be increased, and mass industrial production can be realized.
Owner:QINGDAO JIULONG BIO PHARMA
Method of extracting chondroitin 6-sulfate sodium salt from animal cartilage by solid-phase alkali hydrolysis
The invention discloses a method of extracting chondroitin 6-sulfate sodium salt from animal cartilage by solid-phase alkali hydrolysis; the method replaces existing common liquid-phase alkali-enzymatic hydrolysis by solid-phase alkali hydrolysis; at room temperature, animal cartilage powder is mixed with NaOH and NaCl, reaction is triggered under the action of mechanical force, and the extraction and separation process of chondroitin 6-sulfate sodium salt in solid phase is finished efficiently by making use of the features of the solid phase reaction, such as high selectivity, high yield and good process simplicity; the method has the advantages of good process simplicity, high extraction rate, short production cycle, low solvent usage, and low emission of liquid waste.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Glucosamine chondroitin sulfate salt and preparation method and application thereof
ActiveCN110872361AHigh purityWide crowdOrganic active ingredientsSkeletal disorderPhosphateIon exchange
The invention relates to the industries of functional food and medicine, in particular to a glucosamine chondroitin sulfate salt as well as a preparation method and application thereof. The structuralformula of the glucosamine chondroitin sulfate salt is shown as a formula (1), and the value range of the average polymerization degree n is 5-1000. The glucosamine chondroitin sulfate salt is obtained by performing ion exchange on glucosamine hydrochloride or sulfate or phosphate and chondroitin sulfate sodium, or dialyzing with a mixed solution, or directly precipitating with the mixed solution, , the molecular weights of chondroitin sulfates are all polysaccharides which are obtained through natural extraction, fermentation preparation or degradation, or a synthesis method and accord withthe chondroitin sulfate structures. The chondroitin sulfate and glucosamine are organically combined together, the glucosamine chondroitin sulfate salt is different from an existing compound product of the chondroitin sulfate and the glucosamine, the glucosamine chondroitin sulfate salt does not contain micromolecular inorganic salts or is very low in content of the micromolecular inorganic salts,and the prepared glucosamine chondroitin sulfate salt has a good treatment effect on osteoarthritis model rats in an oral gavage mode; the micromolecular inorganic salts be used for but not limited to joint treatment or auxiliary health care. The glucosamine chondroitin sulfate salt can be widely applied to the fields of functional foods and medicines.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Composition for increasing bone mineral density as well as health care product and preparation method thereof
InactiveCN112957448AAvoid churnPromote hyperplasiaOrganic active ingredientsHydrolysed protein ingredientsIncreased Bone DensityDrug efficiency
The invention relates to a composition for increasing bone mineral density as well as a health care product and a preparation method thereof. The composition is prepared from the following components in parts by mass: 12.8 to 19.2 parts of glucosamine hydrochloride, 10.4 to 15.6 parts of marine fishbone collagen oligopeptide, 6.4 to 9.6 parts of sodium chondroitin sulfate and 4.8 to 7.2 parts of cortex eucommiae extract. The components of the composition cooperate to effectively increase the bone mineral density and prevent osteoporosis, the health care product prepared from the composition has no toxic or side effect, and the effect of increasing the bone mineral density is remarkable. According to the preparation method disclosed by the invention, microcrystalline cellulose and povidone K30 are added, wet granulation is performed, and a required dosage form is processed. By adjusting the proportional relation of raw materials and auxiliary materials and optimizing wet granulation, a good synergistic improvement effect is achieved among the raw material medicines, that is, after the components are combined, the drug effect is remarkably improved compared with that when the components are independently used, and the drug effect is more durable.
Owner:NINGBO YUFANGTANG BIOTECH +1
Chondroitin sulfate sodium salt heat energy circulating device
InactiveCN111701279APlay the function of preheatingGood heat transferRecuperative heat exchangersSolid sorbent liquid separationSludgeHeat conservation
The invention provides a chondroitin sulfate sodium salt heat energy circulating device, which comprises a resin adsorption device, a first connecting pipe, a first valve, a waste liquid tank, a second connecting pipe, a second valve, a storage tank, a third connecting pipe, a third valve, a multi-stage filter, a filter screen, a fourth connecting pipe, a fourth valve, a water suction pump, a heatexchange pipe, a fifth connecting pipe, a heat preservation tank, a temperature control heating pipe, a water outlet pipe, a fifth valve, a heat preservation preheating sleeving shell structure, a movable pushing plate frame structure, an auxiliary driving wheel structure, a sealable discharging pipeline structure, a PLC and a driving switch, wherein one end of the first connecting pipe is connected with a left flange at the lower end of the resin adsorption device, and the other end of the first connecting pipe is connected with an upper flange of the waste liquid tank. The device has the beneficial effects that through the arrangement of the movable pushing plate frame structure, when a movable screw rod moves, a dredging plate is better driven to move, impurities such as sludge in a multistage filter are cleaned, and then the cleaning effect is improved.
Owner:山东冰文生物技术有限公司
Nano-drug complex loaded with sinomenine and preparation method of nano-drug complex
PendingCN113694212AEasy to wrapImprove hydrophilicityMaterial nanotechnologyOrganic active ingredientsChondroitin Sulfate CDrug carrier
The invention relates to the technical field of biological medicines, in particular to a sinomenine-loaded nano-drug complex and a preparation method thereof. The preparation method of the nano-drug complex comprises the following steps: (1) dissolving sinomenine and a nano-drug carrier in an ethanol solution to obtain an intermediate solution; and (2) mixing the intermediate solution with a chondroitin sulfate C sodium salt aqueous solution according to a volume ratio of 1: (9-11), and adjusting the pH value to obtain the nano-drug complex. The nano-drug complex prepared according to the steps is good in compatibility, stable, biodegradable and small in toxic and side effects.
Owner:JINING MEDICAL UNIV
Sodium chondroitin sulfate, chondroitin-sulfate-containing material and processes for producing the same
InactiveUS20060014256A1Short timeLow costOrganic active ingredientsCosmetic preparationsFood additiveFood material
After a solution obtained by extracting protein from cartilages of fishes which are enzyme treated is filtered, alcohol is added to a filtrate to deposit sodium chondroitin sulfate. The sodium chondroitin sulfate manufactured thus can be used for a raw material of medicines, medicine derivatives, medicine additives, cosmetics, and food additives. After a solution obtained by extracting protein which is obtained by enzyme treatment of cartilages of fishes is filtered, the filtrate is dried to manufacture a chondroitin-sulfuric-acid-containing substance. The chondroitin-sulfuric-acid-containing substance manufactured thus is used for, for instance, cosmetics, and food materials.
Owner:NIPPON BARRIER FREE
Method for increasing content of chondroitin sulfate sodium
The invention relates to a method for increasing the content of chondroitin sulfate sodium. Chondroitin sulfate sodium is a mucopolysaccharide substance extracted from animal cartilages such as nasal bones of pigs and the like. The content of the chondroitin sulfate sodium product prepared by a traditional process is 90-95%, and the limit of protein impurities is above 5% while the European Pharmacopoeia stipulates that the content of chondroitin sulfate sodium is not lower than 95% and the limit of protein impurities is not higher than 3%. Visibly, the traditional process can not meet the requirements of the European Pharmacopoeia. In the method, the protein impurities in the chondroitin sulfate sodium product are removed by adopting a method of thermal precipitation. The process is reasonable, is high in removal efficiency, improves the product content and meets the requirements of the European Pharmacopoeia.
Owner:QINGDAO JIULONG BIO PHARMA
Formula food for enhancing bone density of joints and preparation method thereof
The present invention relates to the technical field of food and particularly discloses a formula food for enhancing bone density of joints and a preparation method thereof. The formula food comprisesthe following raw materials in parts by weight: 15-25 parts of chondroitin sulfate, 10-15 parts of D-glucosamine hydrochloride, 5-10 parts of a rhizoma drynariae extract, 4-6 parts of collagen and 5-10 parts of vitamin C. By adding the chondroitin sulfate, the formula food for enhancing the bone density of the joints can stimulate chondrocyte to synthesize collagen, proteoglycan and HA and control formation of new cartilage tissues. A combination of chondroitin sulfate sodium salt, the D-glucosamine hydrochloride and collagen can treat arthritis and relieve symptoms, and the chondroitin sulfate can remove a large amount of Na+ in synovial membranes, promote bone growth and restore damaged cartilages to normal, and by adding the rhizoma drynariae extract, the whole formula food is rich inflavonoids, alkaloids, phenols and other effective ingredients, and has effects of dispersing stasis, stopping pains and setting bones and tendons.
Owner:山东国和堂制药有限公司
Glucosamine chondroitin tablet containing active peptide and organic calcium and preparation method of glucosamine chondroitin tablet
ActiveCN112715943AKeep healthyImprove stabilityOrganic active ingredientsPeptide/protein ingredientsGlucosamine chondroitinGlucosamine / Potassium
The invention provides a glucosamine chondroitin tablet containing active peptide and organic calcium and a preparation method of the glucosamine chondroitin tablet. The glucosamine chondroitin tablet comprises 50 to 250 parts of active peptide, 180 to 550 parts of calcium citrate malate, 80 to 520 parts of glucosamine potassium sulfate, 110 to 290 parts of chondroitin sulfate sodium, 10 to 90 parts of diluent, 30 to 170 parts of dry adhesive, 1 to 20 parts of adsorption hardening agent, 1 to 14 parts of lubricant, 0.05 to 3 parts of anti-sticking agent, 1 to 22 parts of film-forming agent, 0.01 to 7 parts of plasticizer and 0.05 to 5 parts of screening agent. The glucosamine chondroitin tablet disclosed by the invention is good in stability and high in calcium ion human body absorption and utilization rate, and can provide a safe and effective calcium source for patients with osteoporosis; meanwhile, damage to the tabletting mold in the production process is effectively reduced, the production and maintenance cost is reduced, dust on one side during tabletting is reduced, the product yield is increased, and the tabletting device is suitable for industrial large-scale production.
Owner:SHENZHEN TAITAI PHARMA IND +2
A kind of soft tissue repair collagen membrane and preparation method thereof
ActiveCN114053480BImprove uniformityPrevent stratificationTissue regenerationProsthesisTissue repairSlurry coating
The invention relates to a soft tissue repair collagen film and a preparation method thereof. The preparation method comprises the following steps: adding type I collagen to the acetic acid solution to prepare a collagen-acetic acid swelling solution; then adding sodium chondroitin sulfate, stirring evenly to obtain a collagen-chondroitin sulfate sodium slurry; The collagen chondroitin sulfate sodium slurry is defoamed, then freeze-dried and pressed to obtain a collagen basement membrane; the collagen chondroitin sulfate sodium slurry is poured between two layers of the collagen base membrane, and Slurry is applied to make the thickness of the slurry uniform and smooth without air bubbles to obtain a pre-collagen film; the pre-collagen film is firstly subjected to secondary freeze-drying, and then sequentially cross-linked and sterilized to obtain the collagen film for soft tissue repair. Among them, the slurry defoaming process is added to reduce the phenomenon of holes and delamination on the membrane surface caused by air bubbles in the production process; and the use of a slurry coating device greatly improves the uniformity of the membrane thickness and avoids the occurrence of delamination.
Owner:TIANXINFU (BEIJING) MEDICAL APPLIANCE CO LTD
A kind of chondroitin sulfate sodium capsule and preparation method thereof
ActiveCN108159015BSolve the problem of moisture absorptionGood quality and stabilityOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseChondroitin sulfate sodium
The invention discloses a sodium chondroitin sulfate capsule and a preparation method thereof. The sodium chondroitin sulfate capsule comprises following raw materials in parts by weight: 50 to 60 parts of sodium chondroitin sulfate, 30 to 40 parts of corn starch, 3 to 8 parts of low-substituted hydroxypropyl cellulose, 1 to 5 parts of silica, and 1 to 5 parts of talcum powder. The preparation method comprises following steps: (1) drying; (2) mixing; (3) filling; and (4) performing bubble cap and filling capsules in a moisture barrier bag. The problem of moisture absorption of a sodium chondroitin sulfate capsule is solved, the product quality stability is improved, and the product quality can be ensured within the shelf life.
Owner:CHENGDU TONGDE PHARMA
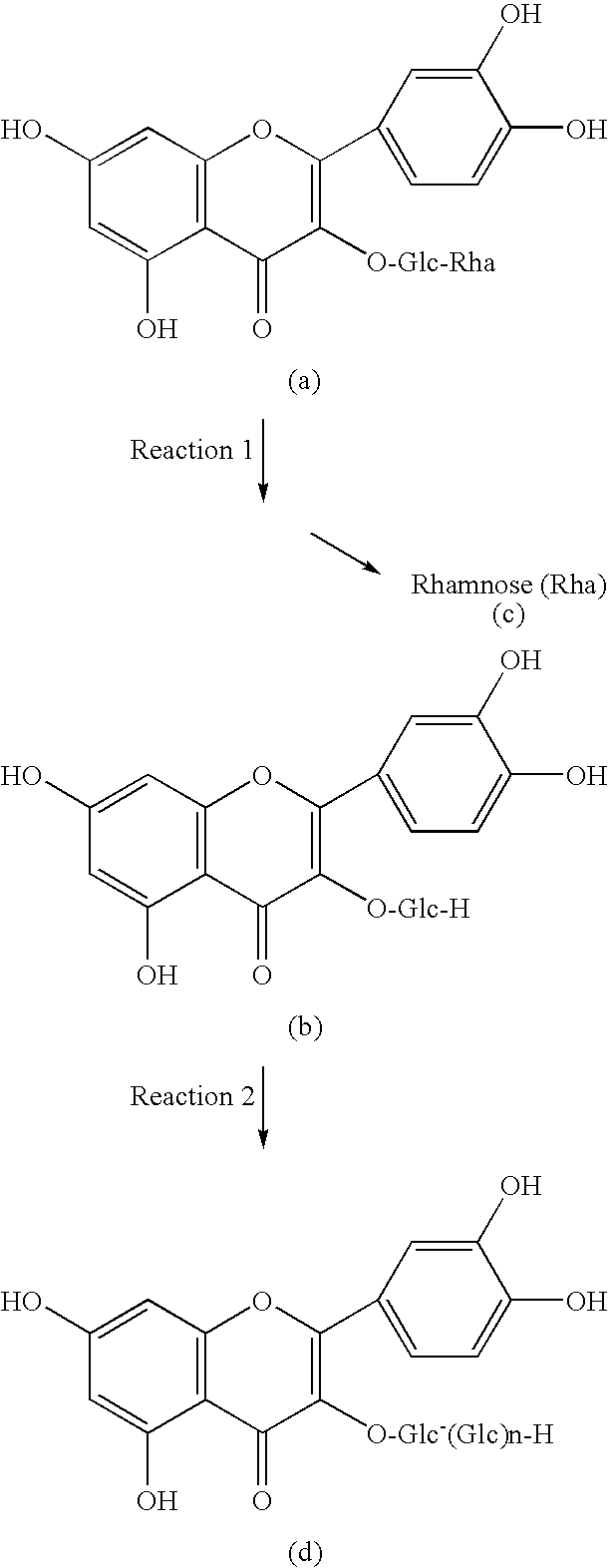
Method for manufacturingα-glycosylisoquercitrin, intermediate product and by-product thereof
The present invention provides a method for producing isoquercitrin, α-glycosylisoquercitrin, and rhamnose, the method comprising a step of naringin-degrading enzyme treatment during the isoquercitrin production from rutin in the presence of an edible component, such as gelatin, wheat gluten, chitosan, lecithin, a glycerol fatty acid ester, xanthan gum, carrageenan, sodium chondroitin sulfate, casein, enzymatically decomposed gelatin, sodium alginate, konjac extract, gellan gum, guar gum, soybean protein, agar, pectin, yeast extract, egg-white peptide, cluster dextrin, gum arabic, arginine, sodium metaphosphate, karaya gum, locust bean gum, sodium pyrophosphate, glucosamine, chitin, sodium glutamate, dextrin, trehalose, or a grain-based food ingredients. According to the present invention, isoquercitrin and α-glycosylisoquercitrin, which are of use as antioxidants, anti-fading agents, flavor change inhibitors, etc., can be produced in enhanced yields.
Owner:SAN EI GEN F F I
Method for purifying chondroitin sulfate sodium salt
The invention discloses a method for purifying chondroitin sulfate sodium salt. The purification method comprises the following steps: (1) dissolving a crude chondroitin sulfate sodium salt in water, adding the crude chondroitin sulfate sodium salt onto a strong acidic cation-exchange resin column, collecting the column liquid, eluting by using purified water, and collecting the eluent for later use; and (2) adding the collected eluent in the step (1) onto a macroporous faintly alkaline cation exchange column, sequentially eluting by using purified water and a sodium chloride solution, collecting the eluent, filtering the eluent, performing ultrafiltration desalting, precipitating by using ethanol, and drying, thereby obtaining the purified chondroitin sulfate sodium salt. The purity of the chondroitin sulfate sodium salt purified by the method disclosed by the invention is over 98.0 percent, the amount of impurities is small, and the protein content can be reduced to be 1.1 percent or below. According to purification in the invention, 100kg of high-purity chondroitin sulfate sodium salt can be obtained per day by virtue of 1000L of resins, and large-scale production can be realized.
Owner:QINGDAO JIULONG BIO PHARMA
Alkali-enzymolysis method for extracting chondroitin sulfate sodium
ActiveCN103665188AOptimize production process conditionsShorten the production cycleChondroitin sulfate sodiumAlkaline hydrolysis
The invention discloses a novel method for extracting chondroitin sulfate sodium, namely an alkali-enzymolysis method for extracting chondroitin sulfate sodium, and mainly solves the problems of complicated production technology processes, larger alkali, acid or salt dosage, higher production cost, longer extraction period, unstable product quality, lower yield and the like for similar products at present. The product mainly adopts animal cartilage as a raw material, and chondroitin sulfate sodium can be extracted from the animal cartilage after alkaline hydrolysis and further enzymolysis; and the color of the product is pure white, the powder is loosened, the yield can be higher than 30%, moreover, the purity is high, and the product meets the oral quality standard. The alkali-enzymolysis method for extracting chondroitin sulfate sodium has the characteristics of simple production process, short period, low cost, simplicity, convenience and easiness in operation, capacities of reducing environment pollution and facilitating environmental protection, and the like.
Owner:QINGDAO JIULONG BIO PHARMA
Preparation method of chondroitin sulfate sodium
The invention discloses a preparation method of chondroitin sulfate sodium. The method comprises the following steps: adding water into a cartilage raw material, cooking, taking out, and pulverizing to obtain cartilage powder; adding mixed powder of sodium hydroxide and silicon dioxide into the cartilage powder, co-grinding, adding a sodium chloride solution, adjusting the pH value, centrifuging and collecting supernatant; carrying out alkali extraction on the precipitate, centrifuging, and collecting the supernatant; mixing the supernatant, adjusting the pH value to 8-10, and then adding alkaline protease for enzymolysis to obtain a chondroitin sulfate solution; adding an adsorbent, keeping standing, carrying out filter pressing, and collecting a filtrate; and adding ethanol with different concentrations into the filtrate by stages for purification to obtain the product. The method can be used to effectively solve the problems of waste, environmental pollution, long extraction time and low product purity caused by a large amount of sodium hydroxide solution in the existing method.
Owner:四川菲德力制药有限公司
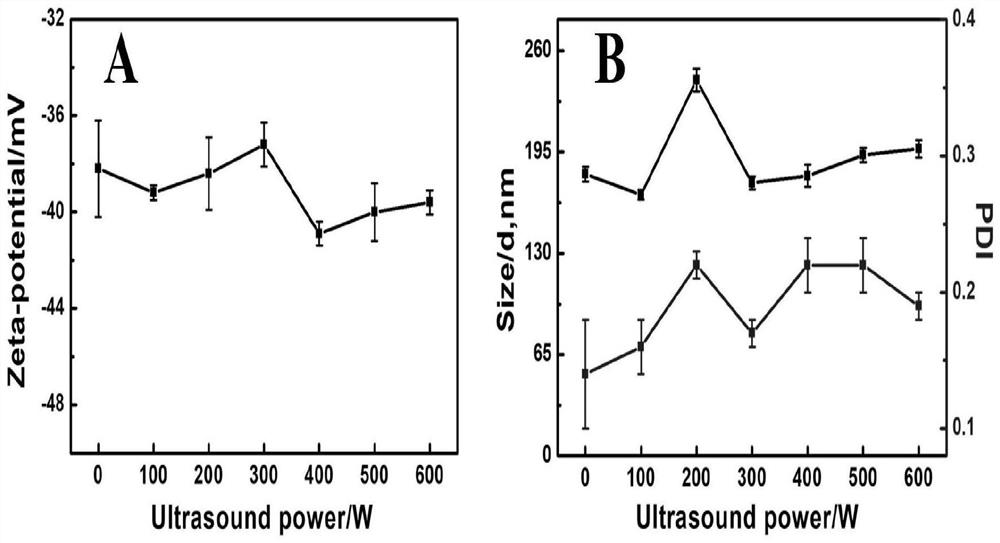
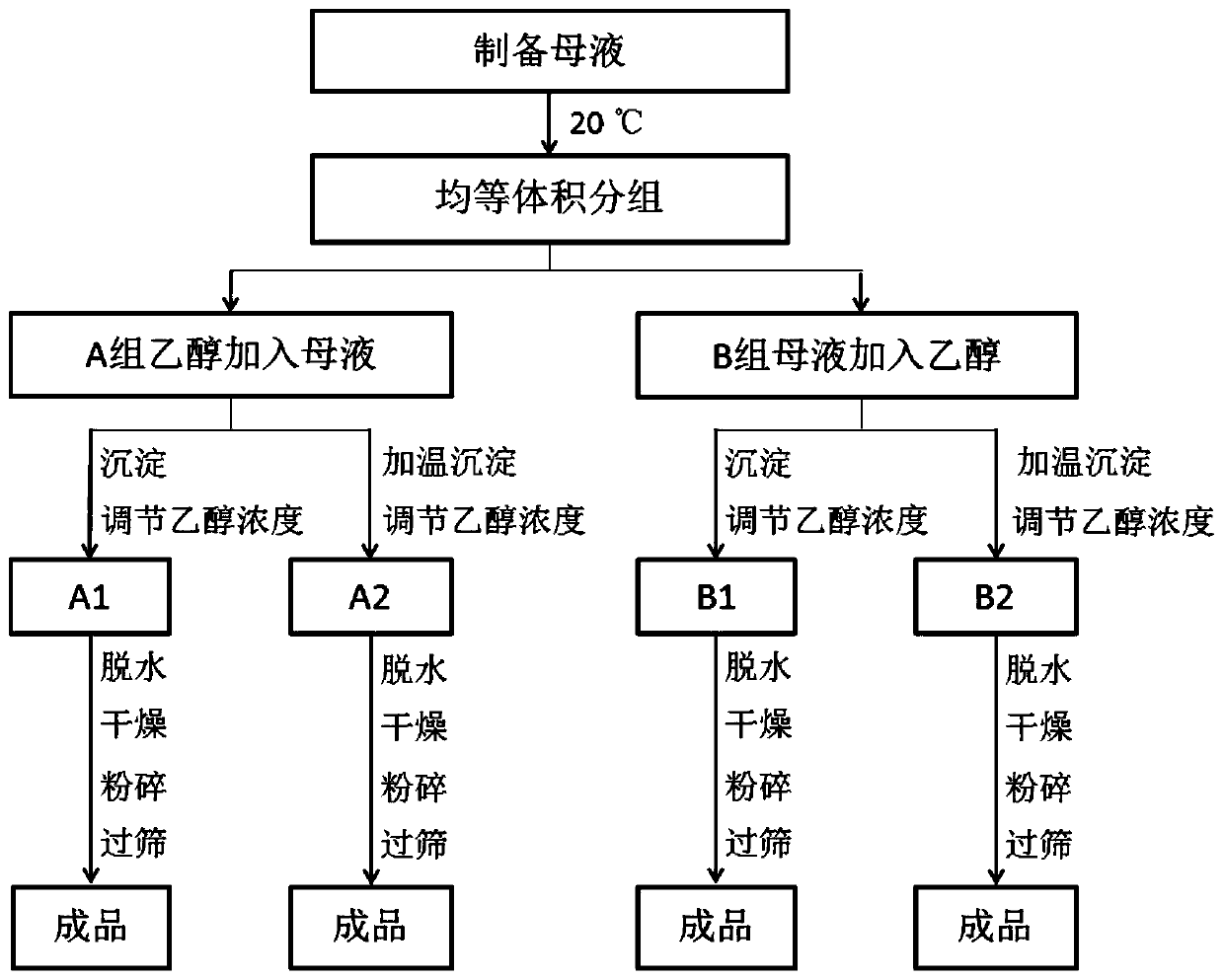
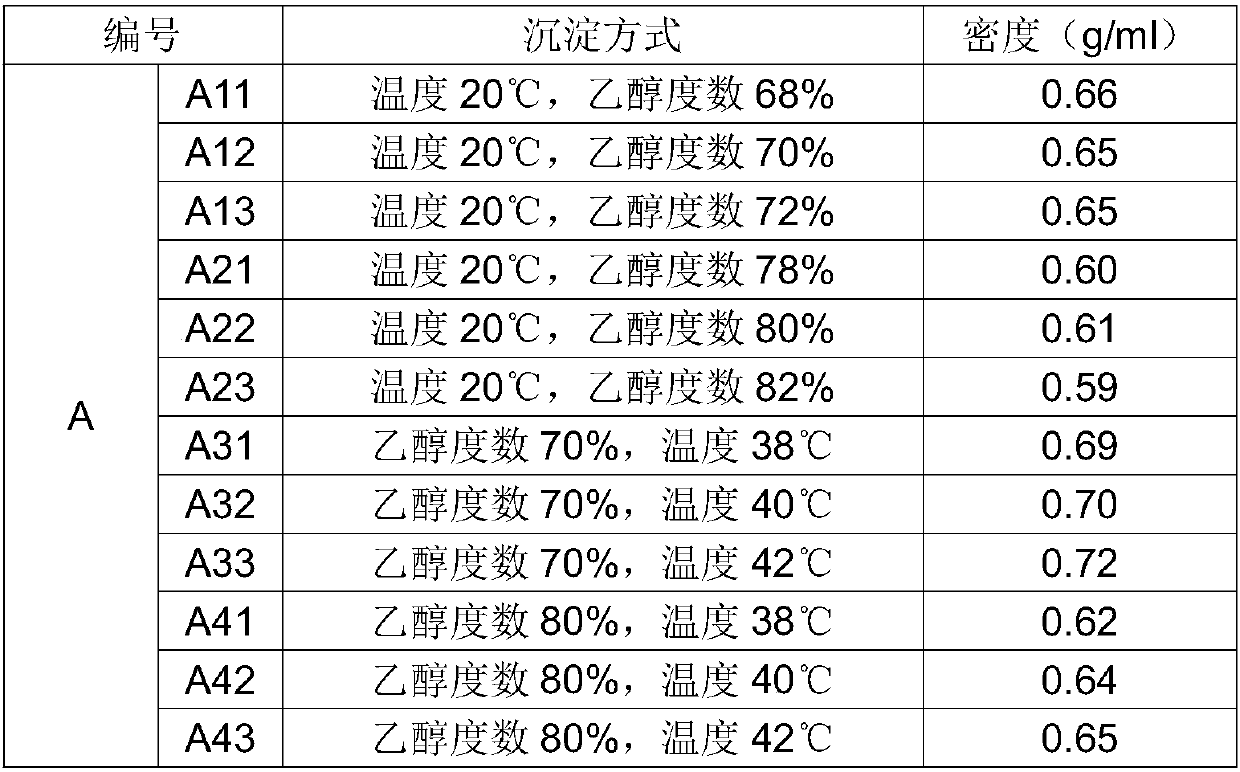
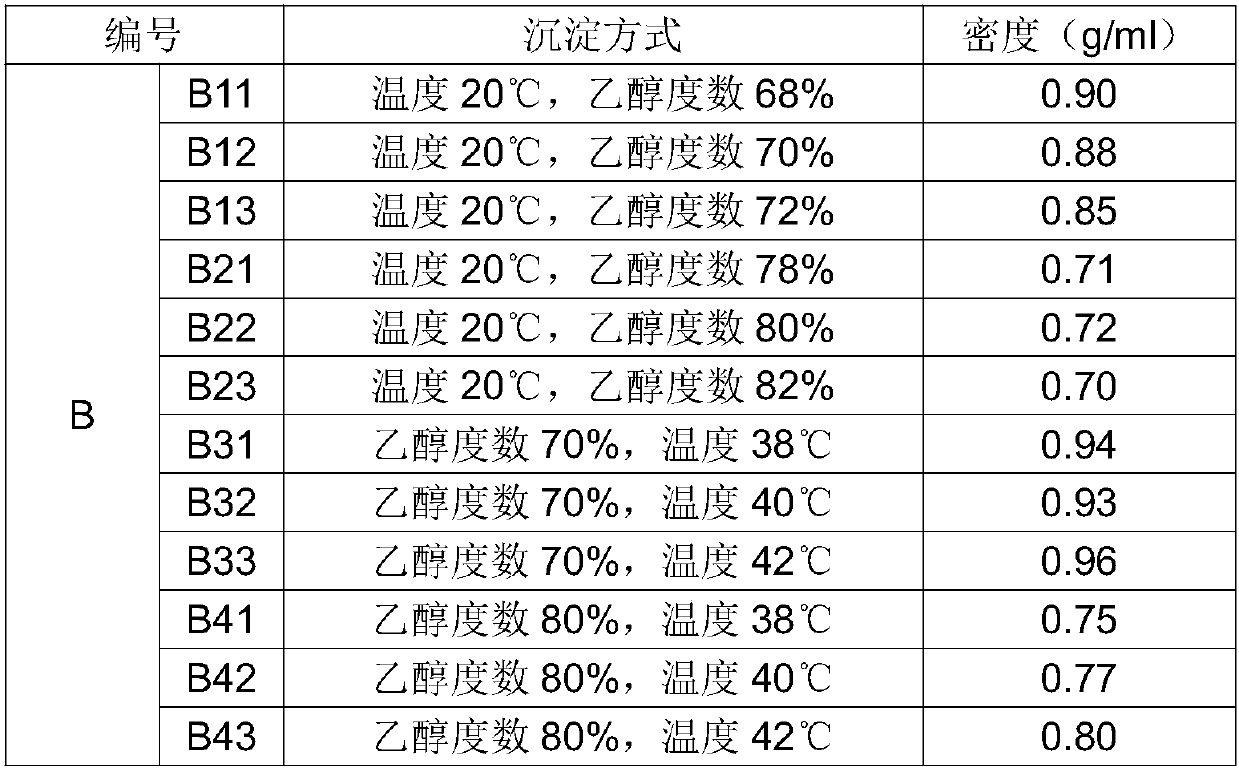
A kind of production technology for controlling the density of chondroitin sulfate sodium
The invention belongs to the field of medicine and particularly discloses a production technology capable of controlling the density of chondroitin sulfate sodium. The technology comprises the steps as follows: mother liquor is prepared and divided into A1, A2, B1 and B2 with equal volumes, and the temperatures of the solutions are adjusted to be 20 DEG C; the A1 and the A2 are precipitated by adding ethanol to the mother liquor, and the concentration of ethanol is adjusted to 68%-82% after precipitation of the A1; the precipitation temperature of the A2 is adjusted to the room temperature of 38-42 DEGC, and the concentration of ethanol is adjusted to 70%-80% after precipitation of A2; the B1 and the B2 are precipitated by adding ethanol to the mother liquor, and the concentration of ethanol is adjusted to 68%-82% after precipitation of the B1; the precipitation temperature of the B2 is adjusted to the room temperature of 38-42 DEGC, and the concentration of ethanol is adjusted to 70%-80% after precipitation of B2; the precipitated solutions are dehydrated, dried, crushed and sieved, and chondroitin sulfate sodium raw materials with different densities are obtained. The technology is reasonable in design, simple and convenient, the densities of the raw materials are easy to control, the cost is low, the yield rate in product preparation production is increased, and the technology is applicable to large-scale industrial production.
Owner:RIZHAO LANSHAN BIOCHEM PROD
Compound brain protein hydrolysate tablet and preparation process thereof
InactiveCN112076171AFast disintegration timeGuaranteed mouthfeelNervous disorderHydrolysed protein ingredientsVitamin b6Sucrose
The invention discloses a compound brain protein hydrolysate tablet and a preparation process thereof, and relates to the technical field of medicine processing. The compound brain protein hydrolysatetablet is prepared from the following components in parts by mass: 60-75 parts of brain protein hydrolysate, 6-7 parts of glutamic acid, 6-7 parts of sodium chondroitin sulfate, 11-13 parts of microcrystalline fibers, 10-12 parts of vitamin B1, 0.1-0.3 part of vitamin B6, 7-9 parts of dextrin, 8-10 parts of calcium carbonate, 10-12 parts of corn starch, 3-5 parts of magnesium stearate for coating, 100-130 parts of sucrose and 60-70 parts of talcum powder. The preparation process comprises the following steps: sequentially granulating the raw materials, drying, granulating and totally mixing,tabletting, coating, and airing, thereby obtaining the compound brain protein hydrolysate tablet. The coating is performed in three steps, namely an isolation layer, a powder coating layer and a colored sugar coating layer. The coating raw materials are prepared from syrup, so that the disintegration time of the coating is accelerated, the drug effect time is prolonged, and the taste of the compound brain protein hydrolysate is guaranteed.
Owner:安徽金太阳生化药业有限公司
Preparation method of multilayer artificial dura mater
The invention relates to a preparation method of a multi-layer artificial dura mater, which comprises the following steps: preparing serous fluid containing chondroitin sulfate sodium and collagen, and sequentially carrying out pre-freezing, freeze drying, high-temperature vacuum crosslinking and sterilization treatment on the serous fluid. According to the method, a special gradient pre-freezing process is adopted, after pre-freezing, the multi-layer artificial dura mater with different densities can be obtained only through one-time freeze drying, and meanwhile process parameters of high-temperature vacuum crosslinking are optimized. The finally prepared multi-layer artificial dura mater is excellent in anti-seepage performance, anti-degradation performance and sewing performance.
Owner:TIANXINFU (BEIJING) MEDICAL APPLIANCE CO LTD
Freeze-dried powder preparation for myopia prevention and eyesight improvement
InactiveCN111956619AHigh activityEffective decontamination and cleaningPowder deliverySenses disorderBetaineOphthalmology
The invention relates to the technical field of eye care products, in particular to a freeze-dried powder preparation for myopia prevention and eyesight improvement. The freeze-dried powder preparation consists of freeze-dried powder and solvent liquid, wherein raw materials of the freeze-dried powder include (a) an active ingredient, (b) an excipient and (c) an emulsifier; the active ingredient comprises sodium hyaluronate, xanthophyll, ascorbic acid, oligopeptide-1, oligopeptide-5 and pyrimidinecarboxylic acid; the excipient comprises mannitol and sodium chondroitin sulfate; the emulsifier comprises hydrogenated lecithin; and the solvent liquid comprises small molecule alcohol, taurine, betaine, retinol, sodium chloride, lysozyme and deionized water. The freeze-dried powder preparation is prepared by dissolving the freeze-dried powder through the solvent liquid. The invention also provides a preparation method of the freeze-dried powder preparation for myopia prevention and eyesightimprovement. Test results show that the freeze-dried powder preparation can effectively moisturize the eyes, relieve and reduce pressure, resist inflammation, activate the eyes, supplement nutrition and enhance immunity, and has a good nursing effect on myopia prevention and eyesight improvement. And the freeze-dried powder preparation is instantly dissolved and used at one time, is non-irritating, has no toxic and side effects and is very safe to use.
Owner:上海旷世医疗管理有限公司
Composition and health-care product for strengthening bones and supplementing calcium and preparation method of health-care product
PendingCN112137087AImprove securityGood bone strengthening and calcium supplementation effectFood shapingNatural extract food ingredientsBiotechnologyGlucosamine / Potassium
The present invention belongs to the field of health-care food and discloses a composition and a health-care product for strengthening bones and supplementing calcium, and a preparation method of thehealth-care product. The composition for strengthening bones and supplementing calcium comprises the following components: calcium carbonate, glucosamine potassium sulfate, a pseudo-ginseng extract, chondroitin sulfate sodium, casein phosphopeptides and a rhizoma drynariae extract. The composition has good safety and also good effects of strengthening the bones and supplementing calcium.
Owner:兰州大得利生物化学制药(厂)有限公司
Preparation method for selenium-rich aquatic animal nutrient
InactiveCN109315635AThe promotion effect is obviousPromote growthLactobacillusAnimal feeding stuffInonotus obliquusWeight gain
The invention relates to a preparation method for a selenium-rich aquatic animal nutrient. The formula (in percentage by weight) consists of 0.8 to 1.8 percent of aspartic acid, 0.9 to 1.9 percent ofdl-Alpha-tocopheryl acetate, 1.2 to 2.2 percent of calcium gluconate, 1.5 to 2.5 percent of bifidobacterium longum, 0.2 to 1.2 percent of ammonium chloride, 1 to 2 percent of sea cucumber extract, 2.5to 3.6 percent of lactobacillus, 2.3 to 3.3 percent of sorbitan monooleate, 2.6 to 3.6 percent of inonotus obliquus extract, 2.2 to 3.2 percent of proteus hauser, 1.7 to 2.7 percent of sodium butyrate, 1.9 to 2.8 percent of protease, 0.3 to 1.3 percent of chondroitin sulfate sodium salt, 0.1 to 1.1 percent of ammonium sulfite, 0.6 to 1.6 percent of sodium carboxymethylcellulose, 0.7 to 1.5 percent of vitamin C, 0.5 to 1.6 percent of potassium sulfate, 1.2 to 2.1 percent of trehalose, 1.1 to 2.2 percent of probiotics, 0.2 to 1.3 percent of selenium yeast and 76.5 to 56.5 percent of water. Theinvention can effectively enable the growth performance of aquatic animals to reach an optimal level, and the specific growth rate, the weight gain rate, the feed utilization rate, the protein utilization rate and the feed protein deposition rate can all reach the maximum.
Owner:CHANGSHA XIEHAOJI BIOENG CO LTD
A kind of pharmaceutical composition for treating osteoporosis and preparation method thereof
ActiveCN104306959BImprove securityPromote healingOrganic active ingredientsPeptide/protein ingredientsBone densityPuerariae Radix extract
The invention discloses a medicine composition for treating osteoporosis and a preparation method thereof. The medicine composition is prepared from collagen, chondroitin sulfate sodium salt, calcium carbonate, rhizoma drynariae extract, kudzu vine root extract, D-glucosamine hydrochloride and pseudo-ginseng. The medicine composition can be used for treating the pathogenesis, namely destruction of bone microstructure, of osteoporosis, is capable of solving the problem of bone density abnormality and radically preventing and treating osteoporosis; and the medicine composition can be used for relieving symptoms of ostealgia of osteoporosis patients, healing of bone fracture can be promoted, and the living quality of osteoporosis patients can be improved.
Owner:HEBEI YUZHILIN PHARMA
Preparation method of glucosamine composite functional tablet
PendingCN114343184AIncreased Glucosamine ContentPromote dissolutionFood shapingNatural extract food ingredientsVitamin CStearic acid
Owner:JIANGSU ALAND NOURISHMENT
Method for reducing residual ethanol of chondroitin sulfate sodium
The present invention relates to a method for reducing the residual ethanol of chondroitin sulfate sodium, wherein chondroitin sulfate sodium is a mucopolysaccharide substance extracted from pig nasalbone and other animal cartilages, and mainly precipitation purification and dewatering is performed with ethanol. According to the present invention, mainly thermal precipitation and dewatering wit ethanol is performed to reduce the ethanol content in chondroitin sulfate and reduce the residual ethanol; and the method has characteristics of simple process, mild condition, safe production, low residue ethanol content, improved chondroitin sulfate yield and improved purity of chondroitin sulfate, and is the process suitable for large-scale industrial production.
Owner:QINGDAO JIULONG BIO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com