Tissue-guided regenerated collagen membrane and preparation method thereof
A technology that guides regeneration and collagen membranes, applied in medical science, surgery, etc., can solve problems such as unsuitable pore size and porosity, incomplete removal of immunogens, immune reactions, etc., to achieve long degradation cycle, excellent tissue repair effect, comprehensive effect comprehensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
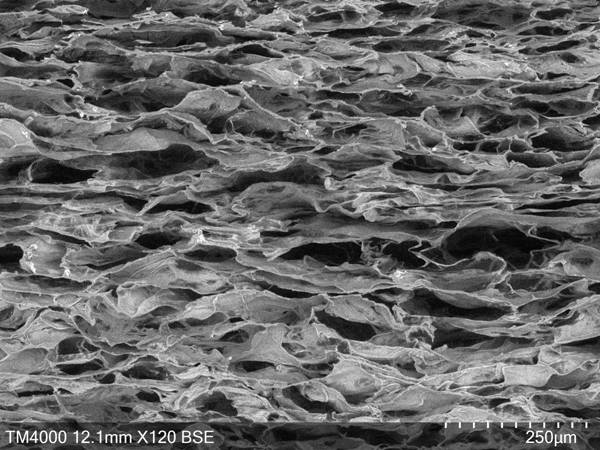
Image
Examples
Embodiment 1
[0025] This embodiment provides a method for preparing a tissue-guided regeneration collagen membrane, comprising the following steps:
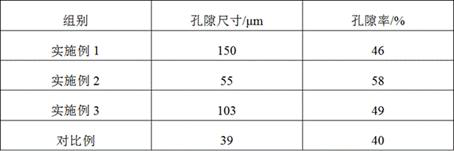
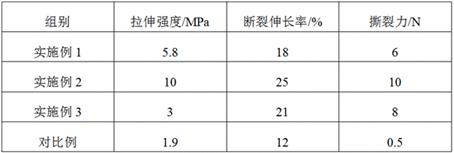
[0026] (1) Swell type I collagen with a purity of 90% in an acetic acid solution with a concentration of 0.4% (v / v), and prepare it to obtain a collagen-acetic acid swelling solution with a concentration of 0.3% (w / v) Add chondroitin sulfate sodium and stir evenly, the concentration of chondroitin sulfate sodium is 0.02% (w / v), and after completion, collagen-chondroitin sulfate sodium slurry is obtained;
[0027] (2) Pour the collagen-sodium chondroitin sulfate slurry into a stainless steel freeze-drying tray to make a thickness of 1 mm, and then place the freeze-drying tray in a vacuum freeze dryer for vacuum freeze-drying to obtain a dried membrane;
[0028] (3) Mix the dry membrane with a cross-linking solution with a concentration of 0.01% (v / v), and the ratio of the area of the dry membrane to the volume of the cross-linking solution i...
Embodiment 2
[0031] This embodiment provides a method for preparing a tissue-guided regeneration collagen membrane, comprising the following steps:
[0032] (1) Swell type I collagen with a purity of 92% in an acetic acid solution with a concentration of 0.7% (v / v), and prepare it to obtain a collagen-acetic acid swelling solution with a concentration of 1.0% (w / v) Add chondroitin sulfate sodium and stir evenly, the concentration of chondroitin sulfate sodium is 0.05% (w / v), and after completion, collagen-chondroitin sulfate sodium slurry is obtained;
[0033] (2) Pour the collagen-chondroitin sulfate sodium slurry into a stainless steel freeze-drying tray to make a thickness of 10 mm, and then place the freeze-drying tray in a vacuum freeze dryer for vacuum freeze-drying to obtain a dried membrane ;
[0034] (3) Mix the dry membrane with a cross-linking solution with a concentration of 1% (v / v), and the ratio of the area of the dry membrane to the volume of the cross-linking solution i...
Embodiment 3
[0037] This embodiment provides a method for preparing a tissue-guided regeneration collagen membrane, comprising the following steps:
[0038] (1) Swell type I collagen with a purity of 91% in an acetic acid solution with a concentration of 0.5% (v / v), and prepare it to obtain a collagen-acetic acid swelling solution with a concentration of 0.6% (w / v) Add chondroitin sulfate sodium and stir evenly, the concentration of chondroitin sulfate sodium is 0.03% (w / v), and after completion, collagen-chondroitin sulfate sodium slurry is obtained;
[0039] (2) Pour the collagen-chondroitin sulfate sodium slurry into a stainless steel freeze-drying tray to make the thickness 5mm, then place the freeze-drying tray in a vacuum freeze dryer for vacuum freeze-drying to obtain a dried membrane;
[0040] (3) Mix the dry membrane with the cross-linking solution with a concentration of 0.5% (v / v), and the ratio of the area of the dry membrane to the volume of the cross-linking solution is 2.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com