A kind of production technology for controlling the density of chondroitin sulfate sodium
A kind of chondroitin sulfate sodium and production process technology, which is applied in the field of medicine, can solve the problems affecting the molding of tablets or capsules, waste of production resources, etc., and achieve the effects of easy control, saving production costs, and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
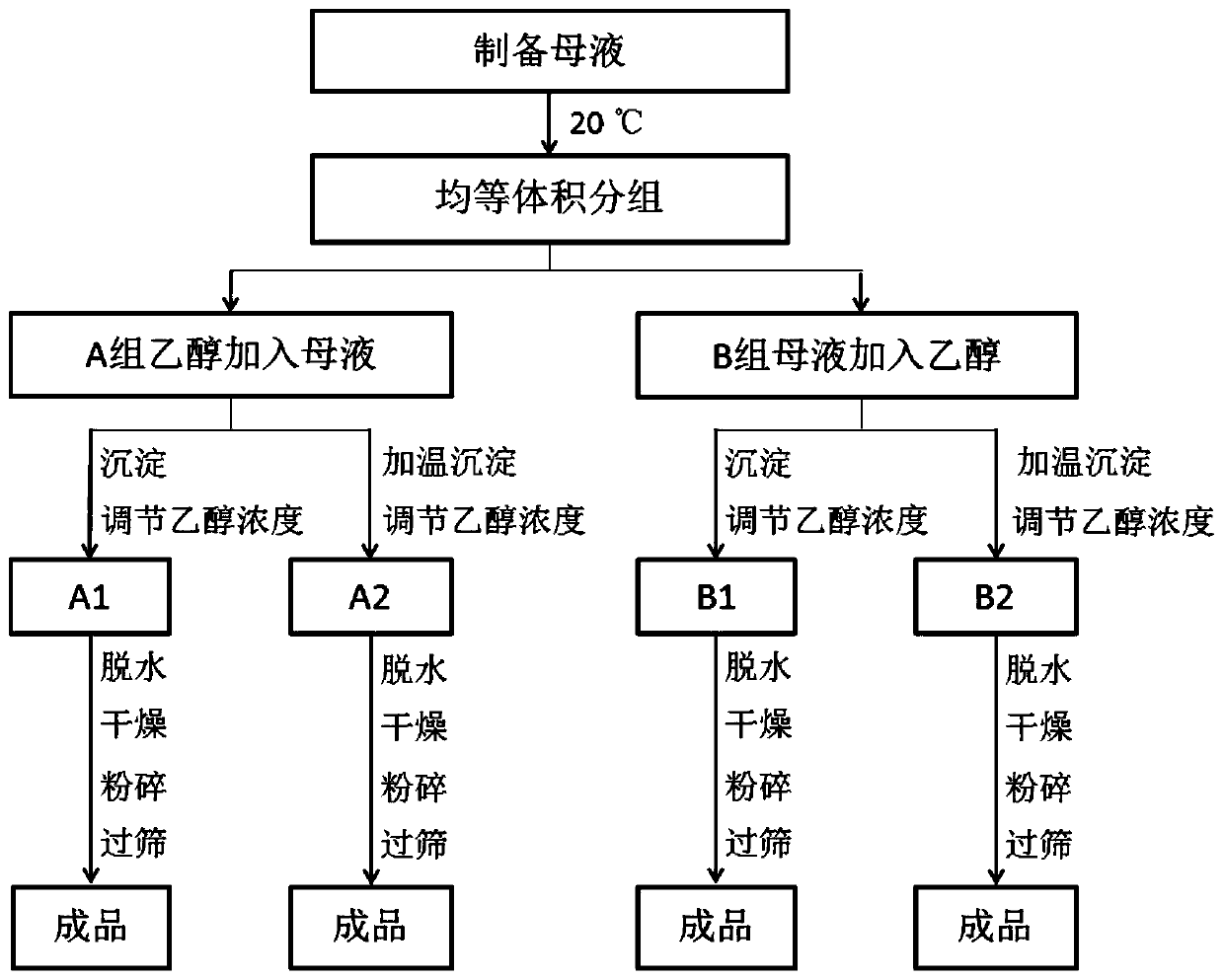
[0058] A production process for controlling the density of chondroitin sulfate sodium, comprising:
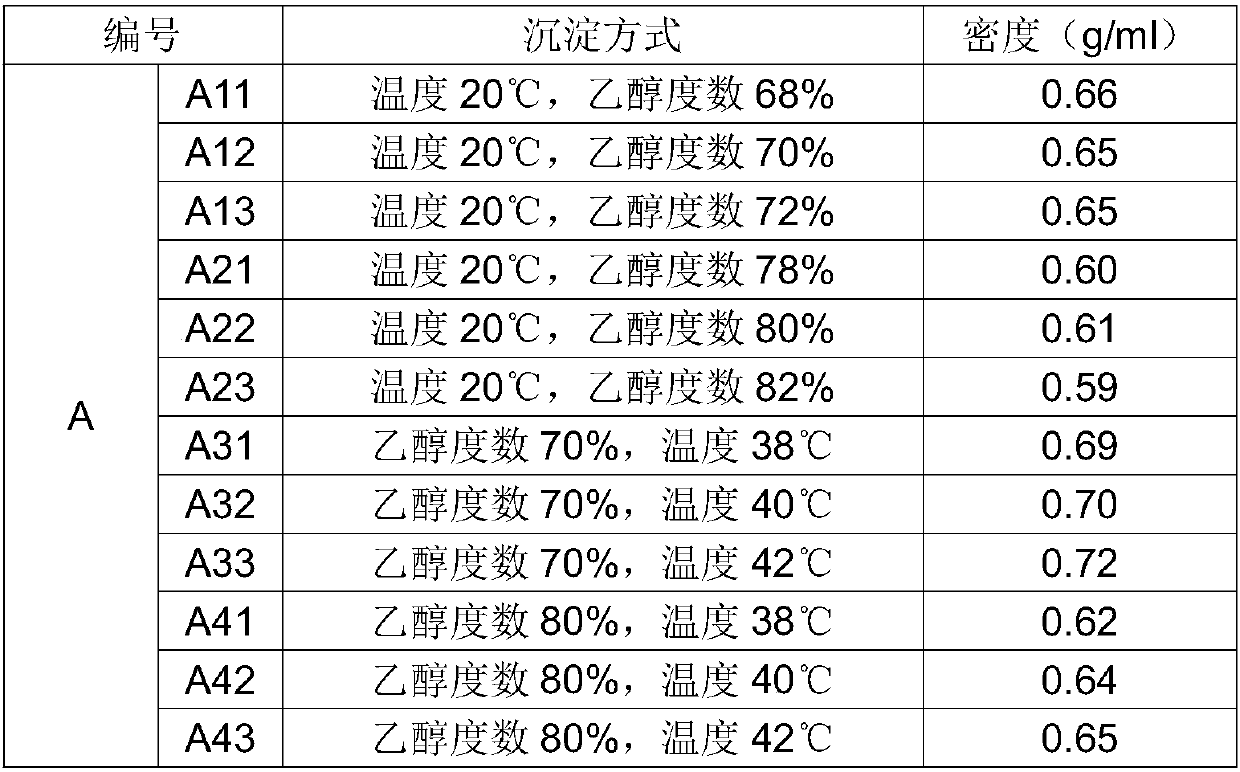
[0059] T1. Take 12 kg of chondroitin sulfate sodium sample and dissolve it in water at a ratio of 1:10, stir evenly, and make a mother liquor, which is recorded as group A, and divided into 12 parts by volume, respectively recorded as A11, A12, A13, A21, A22 , A23, A31, A32, A33, A41, A42, A43, adjust each solution to be 20°C;
[0060] T2, above-mentioned each solution selects ethanol to add the precipitation mode of mother liquor, the ethanol concentration after the precipitation of adjustment A11 is 68%, the ethanol concentration after the precipitation of A12 is 70%, the ethanol concentration after the precipitation of A13 is 72%, the ethanol concentration after the precipitation of A21 The ethanol concentration after the precipitation of A22 is 80%, the ethanol concentration after the precipitation of A23 is 82%, the ethanol concentration after the precipitation of A31 is 7...
Embodiment 2
[0067] A production process for controlling the density of chondroitin sulfate sodium, comprising:
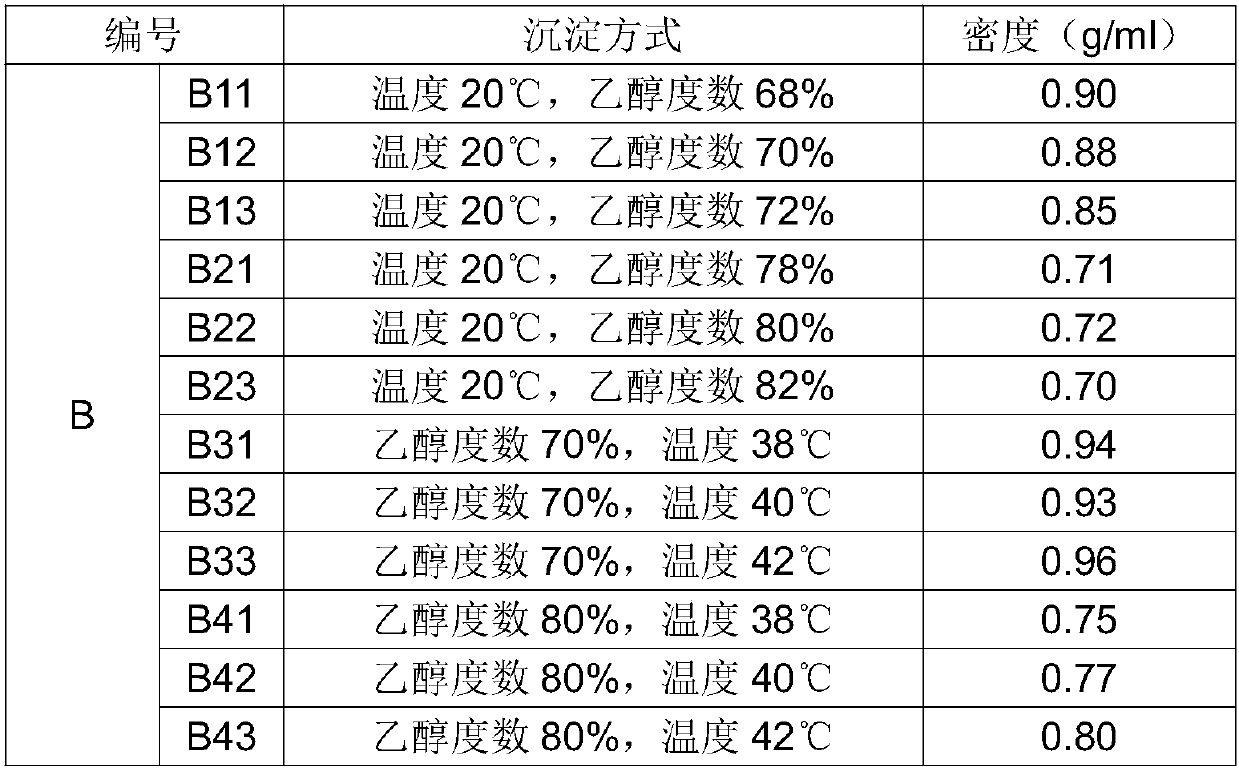
[0068] S1. Take 60 kg of chondroitin sulfate sodium sample and dissolve it in water at a ratio of 1:10, stir evenly, and make mother liquor, which is recorded as group B, and divided into 12 parts by volume, respectively recorded as B11, B12, B13, B21, B22 , B23, B31, B32, B33, B41, B42, B43, adjust each solution to be 20°C;
[0069] S2, above-mentioned each solution selects mother liquor to add the precipitation mode of ethanol, the ethanol concentration after the precipitation of adjustment B11 is 68%, the ethanol concentration after the precipitation of B12 is 70%, the ethanol concentration after the precipitation of B13 is 72%, the ethanol concentration after the precipitation of B21 The concentration of ethanol after precipitation of B22 is 80%, the concentration of ethanol after precipitation of B23 is 82%, the concentration of ethanol after precipitation of B31 is 70%, t...
Embodiment 3
[0076] A customer needs 100kg of chondroitin sulfate sodium raw materials with a density of 0.60±0.05g / ml and 0.80±0.05g / ml respectively for the preparation of capsules and tablets. The process for producing chondroitin sulfate sodium according to the customer’s requirements is as follows:
[0077] 1. Dissolve 250kg of sodium chondroitin sulfate sample in 2500L of water and stir evenly;
[0078] 2. Use a pump to transfer 1250L of mother liquor to the precipitating tank A filled with ethanol, adjust the ethanol concentration to 80%, and the precipitation temperature to 20°C;
[0079] 3. Transfer the remaining 1250L mother liquor to another precipitation tank B, add ethanol and adjust the ethanol concentration to 75%, and the precipitation temperature to 20°C.
[0080] 4. Suction filtration, dehydration, and drying of the materials in the two sedimentation tanks A and B to obtain the bulk drug of chondroitin sulfate sodium; wherein the weight of product A is 121kg, and the weigh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com