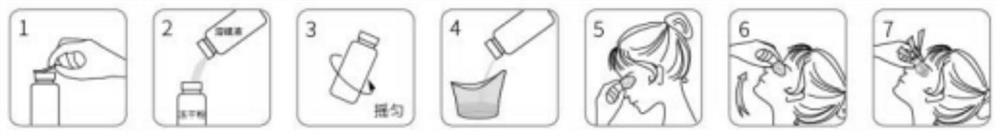
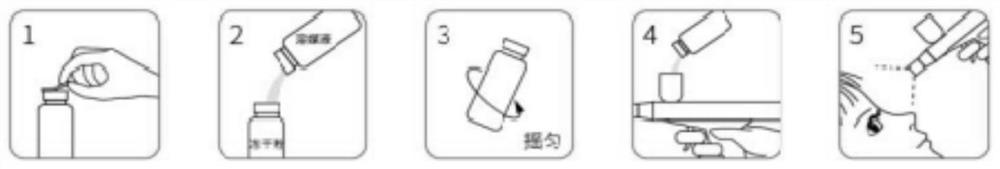
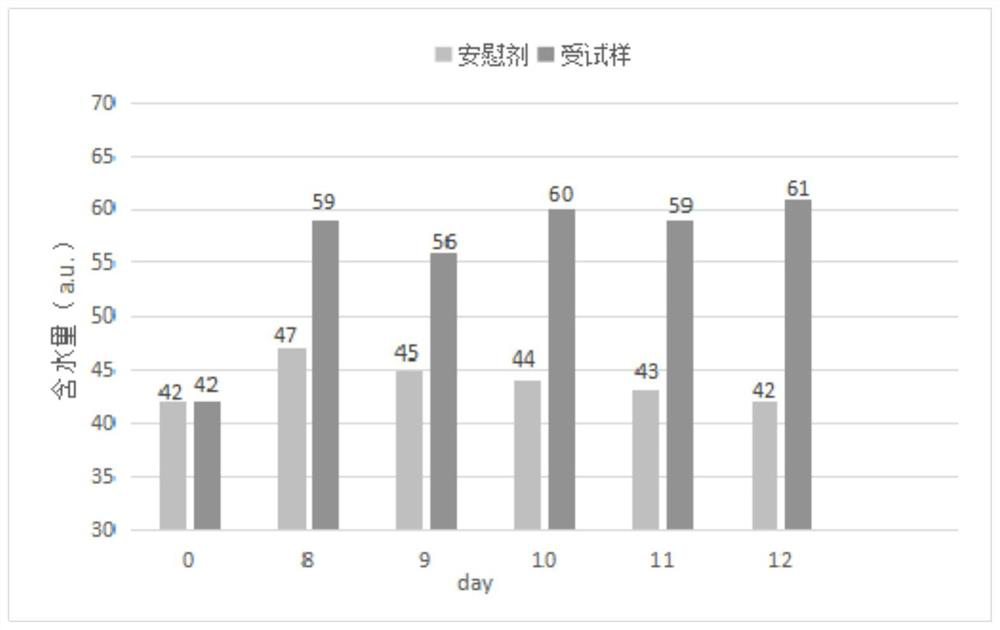
Freeze-dried powder preparation for myopia prevention and eyesight improvement
A freeze-dried powder and preparation technology, applied in biochemical equipment and methods, freeze-dried transportation, medical preparations of non-active ingredients, etc., to achieve safe use, good nursing effects, and immune enhancement effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0081] As a specific embodiment, a third aspect of the present invention provides a method for preparing the freeze-dried powder and the vehicle, including the following specific steps:
[0082] 1. Preparation of freeze-dried powder:
[0083] (1) At room temperature, weigh each component according to the formula, put it in its own container, mark it and put it on the feeding cart according to the order of feeding.
[0084] (2) At room temperature, add deionized water into the vacuum homogeneous emulsification pot, heat to 85∽90°C, keep warm for 30 minutes to sterilize, then cool to 80°C, add mannitol, stir to dissolve evenly.
[0085] (3) Cool to 50°C while stirring, add sodium hyaluronate and sodium chondroitin sulfate in turn, stir for 10 minutes, mix well and dissolve evenly. Add hydrogenated lecithin, stir for 10 minutes, fully mix and dissolve, and beat for 4 minutes.
[0086] (4) Cool to 40°C, add lutein, stir for 10 minutes, and homogenize for 4 minutes to fully mix and...
Embodiment 1
[0107] Embodiment 1 provides a kind of lyophilized powder preparation for the prevention of myopia and vision improvement, which consists of two parts of lyophilized powder and solvent solution, and the composition of its mass percentage is respectively:
[0108] Freeze-dried powder: based on the total weight of the freeze-dried powder, including 3.0% mannitol, 0.1% hydrogenated lecithin, 0.05% sodium hyaluronate, 0.02% sodium chondroitin sulfate, 0.01% lutein, and 0.05% ascorbic acid , tetrahydromethyl pyrimidine carboxylic acid 0.5%, oligopeptide-1 0.0005%, oligopeptide-5 0.0005%, deionized water balance.
[0109] Solvent solution: based on the total weight of the solvent solution, including 1% glycerin, 0.5% betaphylline, 0.2% taurine, 0.001% lysozyme, 0.02% retinol, 0.01% sodium chloride, and the balance of deionized water .
[0110] The preparation method is as follows:
[0111] Freeze-dried powder preparation:
[0112] (1) At room temperature, weigh each component acc...
Embodiment 2
[0131] Embodiment 2 provides a kind of lyophilized powder preparation for myopia prevention and visual acuity improvement, which is composed of lyophilized powder and solvent solution, and the composition of its mass percentage is respectively:
[0132] Freeze-dried powder: based on the total weight of the freeze-dried powder, including 4.0% mannitol, 0.3% hydrogenated lecithin, 0.07% sodium hyaluronate, 0.03% sodium chondroitin sulfate, 0.03% lutein, and 0.1% ascorbic acid , tetrahydromethylpyrimidinecarboxylic acid 1.0%, oligopeptide-1 0.0007%, oligopeptide-5 0.0007%, and the balance of deionized water.
[0133] Solvent solution: based on the total weight of the solvent solution, including 2% glycerin, 1% betaphylline, 0.3% taurine, 0.002% lysozyme, 0.03% retinol, 0.02% sodium chloride, and the balance of deionized water .
[0134] The preparation method of the freeze-dried powder preparation is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com