Glucosamine chondroitin calcium tablet and preparation method thereof
A technology of chondroitin and chondroitin sulfate sodium, which is applied in bone diseases, sugar-coated pills, pharmaceutical formulas, etc., can solve the problems of insufficient hardness of the composition, easy oxidation of the composition, and insufficient stability of performance, so as to achieve stable drug effect and high product quality. Guaranteed quality and scientific design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
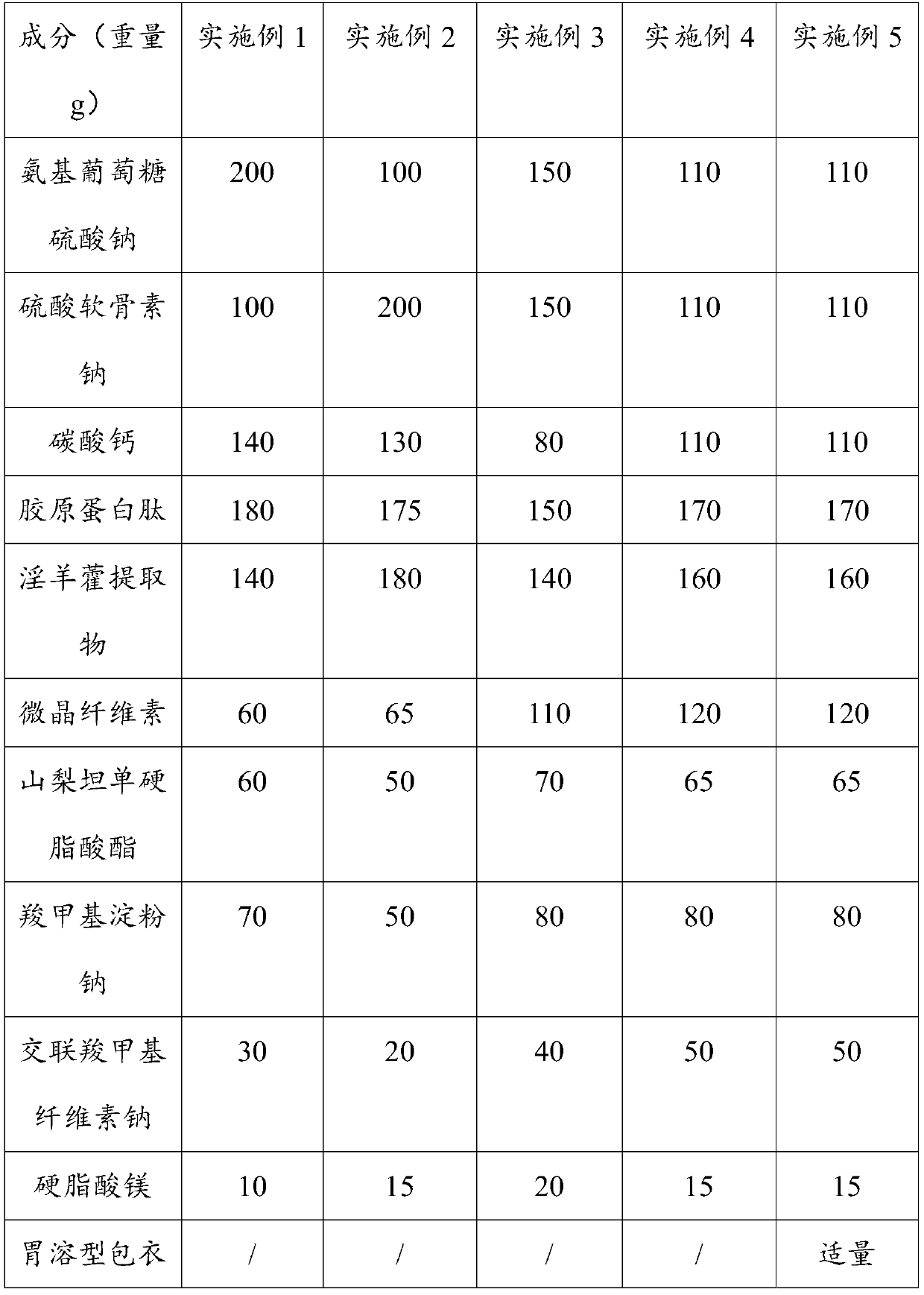
[0041] Embodiments 1 to 5, Glucosamine Chondroitin Calcium Tablets of the Present Invention and Preparation Method The formulations of the Glucosamine Chondroitin Calcium Tablets of Embodiments 1 to 5 of the present invention are shown in Table 1:
[0042] Table 1
[0043]
[0044]
Embodiment 1~4
[0045] Embodiment 1~4, the preparation method of glucosamine chondroitin calcium tablet
[0046] S1. Sieve: Weigh the glucosamine sodium sulfate, sodium chondroitin sulfate, calcium carbonate, collagen peptide, epimedium extract, microcrystalline cellulose, and sorbitan monostearate of Examples 1 to 4, respectively , sodium carboxymethyl starch, croscarmellose sodium and magnesium stearate are respectively passed through an 80-mesh sieve, and set aside;
[0047] S2. Mixing: the glucosamine sodium sulfate, sodium chondroitin sulfate, calcium carbonate, collagen peptide, epimedium extract, microcrystalline cellulose, sorbitan monostearate and sodium carboxymethyl starch obtained in step S1 Mix with each other for 30 minutes to get mixed powder;
[0048] S3. Granulation: add 15 mL of ethanol solution with a volume fraction of 95% to the mixed powder, pass through a 20-mesh sieve and granulate to obtain wet granules, and dry the wet granules at 55°C until the moisture content is <7...
Embodiment 5
[0050] Embodiment 5, the preparation method of glucosamine chondroitin calcium tablet
[0051] S1. Sieving: Take respectively the glucosamine sulfate sodium, chondroitin sulfate sodium, calcium carbonate, collagen peptide, Epimedium extract, microcrystalline cellulose, sorbitan monostearate, carboxylate Methyl starch sodium, croscarmellose sodium and magnesium stearate are respectively passed through 80 mesh sieves, and set aside;
[0052] S2. Mixing: the glucosamine sodium sulfate, sodium chondroitin sulfate, calcium carbonate, collagen peptide, epimedium extract, microcrystalline cellulose, sorbitan monostearate and sodium carboxymethyl starch obtained in step S1 Mix with each other for 30 minutes to get mixed powder;
[0053] S3. Granulation: add 15 mL of ethanol solution with a volume fraction of 95% to the mixed powder, pass through a 20-mesh sieve and granulate to obtain wet granules, and dry the wet granules at 55°C until the moisture content is <7%;
[0054] S4. Tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com