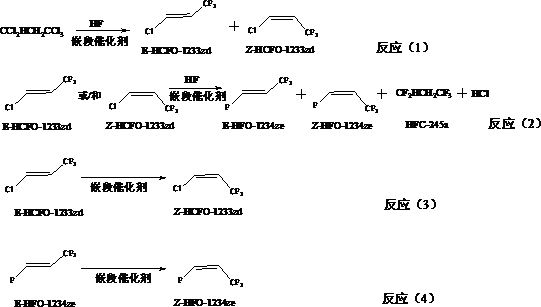
Preparation method of Z-1-halogen-3, 3, 3-trifluoropropene
A technology of trifluoropropene and Z-1-, which is used in the preparation of halogenated hydrocarbons, halogen substitution preparation, organic chemical methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
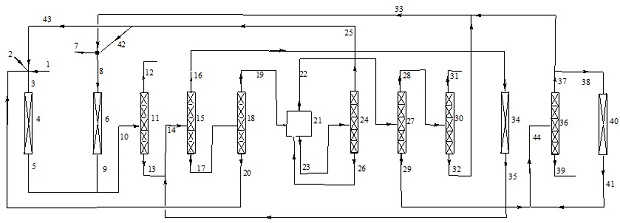
Image
Examples
Embodiment 1
[0052] Preparation of block catalyst: (1) Dissolve tungsten trichloride in water, then dropwise add 10% by mass ammonia water to completely precipitate metal ions, adjust the pH value to 7.0-9.0, and make it fully precipitate under stirring conditions , aged for 24 hours, filtered the formed slurry, then dried at 150°C for 18 hours, pulverized the solid, and pressed into shape to obtain a carrier precursor; the obtained carrier precursor was roasted at 400°C under a nitrogen atmosphere After 18 hours, it was activated at 300°C for 18 hours with a mixed gas consisting of hydrogen fluoride and nitrogen with a molar ratio of 1:2 to obtain a carrier, which was confirmed to be tungsten oxyfluoride by XPS detection; (2) in dry, high-purity In nitrogen or helium or argon atmosphere, according to 20% SbF in block catalyst 5 Composed of 80% tungsten oxyfluoride mass percentage content, the precursor of the active component SbCl 5 Coating on tungsten oxyfluoride to obtain a catalyst pr...
Embodiment 2
[0055] The same operation as in Example 1, the difference is that the block catalyst is made of 30%SbF 5 Composed with 70% tungsten oxyfluoride, and the reaction temperature was changed to 100°C, the reaction result was: the conversion rate of 1,1,1,3,3-pentachloropropane was 54.6%, E-1-chloro-3,3, The selectivity of 3-trifluoropropene was 99.1%, and the selectivity of Z-1-chloro-3,3,3-trifluoropropene was 0.9%.
Embodiment 3
[0057] The same operation as in Example 1, the difference is that the block catalyst is made of 25%SbF 5 Composed with 75% tungsten oxyfluoride, and the reaction temperature was changed to 150°C, the reaction result was: the conversion rate of 1,1,1,3,3-pentachloropropane was 71.6%, E-1-chloro-3,3, The selectivity of 3-trifluoropropene was 97.4%, and the selectivity of Z-1-chloro-3,3,3-trifluoropropene was 2.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com