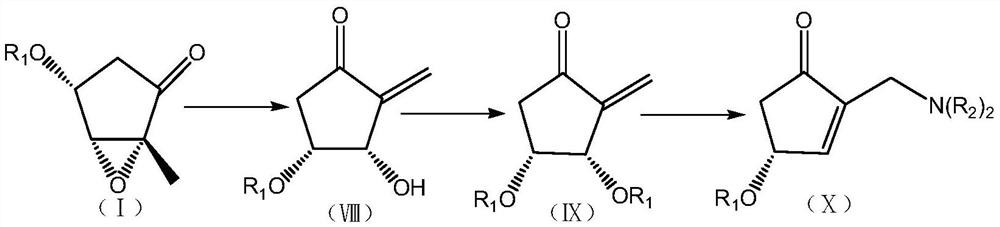
Novel preparation method and intermediate of prostaglandin
A technology for prostaglandins and compounds, applied in the field of chemical synthesis, can solve the problems affecting the application of prostaglandins, the complex preparation process of intermediate VIII, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
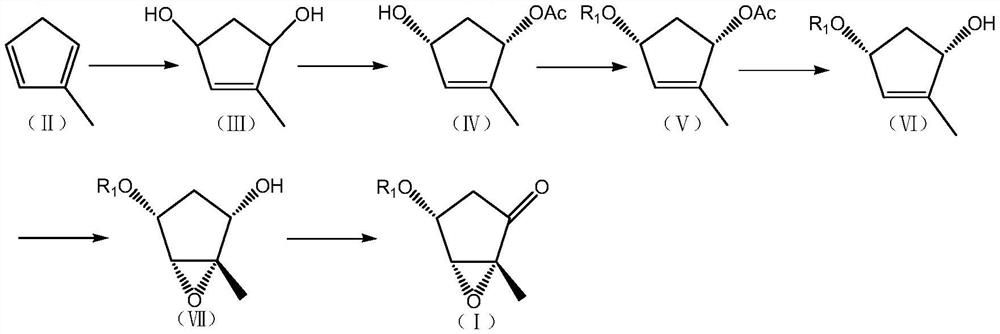
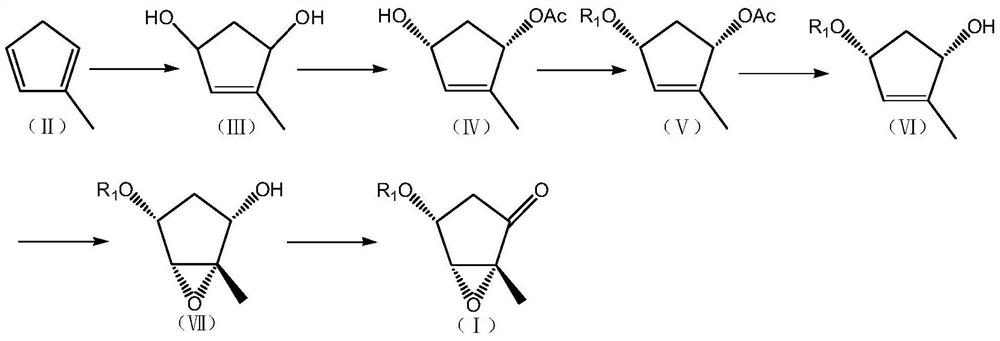
[0114] The preparation of the compound shown in embodiment 1. structural formula (III)
[0115]
[0116] The preparation method of 2-methylcyclopentadiene shown in structural formula (II) can be referred to Tetrahedron.,1965,21,2313-2327, and it can obtain monomer by pyrolysis of the methylcyclopentadiene dimer of commercialization supply , so that the current production and current use
[0117]
[0118] Dissolve the compound represented by structural formula (II) (80.1g, 1.0mol) in MeOH (400.0ml), add rose bengal (8.0g), cool down to -30°C, feed oxygen, and keep warm at -30°C under light for reaction 2 hours, then overnight at room temperature. Add thiourea (76.0 g, 1.0 mol) and react at room temperature for 6 h. Post-processing: the reaction solution was washed into ice water, extracted 3 times with dichloromethane, the dichloromethane layers were combined, washed with water and saturated brine, layered, the organic layer was dried over anhydrous magnesium sulfate, f...
Embodiment 2
[0121] The preparation of the compound shown in embodiment 2. structural formula (IV)
[0122]
[0123] Dissolve the compound (57.0g, 0.5mol) shown in structural formula (III) in THF (350.0ml), stir to dissolve, add vinyl acetate (100.0ml), then add lipase AK (2.8g, Amano Enzyme Co., Ltd. ). After the addition, react at room temperature for 7 hours. Post-processing: filter the reaction solution, concentrate the filtrate, dissolve the residual oily substance with ethyl acetate, wash with water and saturated brine, separate layers, dry the organic layer with anhydrous magnesium sulfate, filter, and concentrate the filtrate to dryness to obtain the product: 71.8g (Yield 92.0%).
[0124] [a] 25 D =-68.6° (C=1.0, CHCl 3 )
[0125] 1 H-NMR (CDCl 3 )δ1.660(dt,1H),δ1.713(s,3H),δ2.063(s,3H),δ2.850(dt,1H),δ4.700-4.750(m,1H),δ5. 470-5.530(m,1H), δ5.970-6.010(m,1H).
[0126] MS (ES + ) 156.1.
Embodiment 3
[0127] The preparation of the compound shown in embodiment 3. structural formula (V) (R 1 = tert-butyldimethylsilyl)
[0128]
[0129] Dissolve the compound (46.8g, 0.3mol) shown in structural formula (IV) in DMF (468.0ml), stir to dissolve, add imidazole (61.0g, 0.9mol), add tert-butyldimethylsilyl chloride (67.8 g, 0.45mol), the addition was completed, and the reaction was carried out at room temperature for 3 hours. Post-processing: wash the reaction solution into ice water, extract 3 times with ethyl acetate, combine the ethyl acetate layers, wash with water in turn, wash with saturated brine, separate layers, dry the organic layer with anhydrous magnesium sulfate, filter, and concentrate the filtrate to Dry to obtain oil: 79.5 g (98.0% yield).
[0130] 1 H-NMR (CDCl 3 )δ0.170(s,6H),δ0.971(s,9H),δ1.640(dt,1H),δ1.721(s,3H),δ2.120(s,3H),δ2.870( dt, 1H), δ4.550-4.610 (m, 1H), δ5.560-5.620 (m, 1H), δ6.010-6.140 (m, 1H).
[0131] MS (ES + )270.65.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com