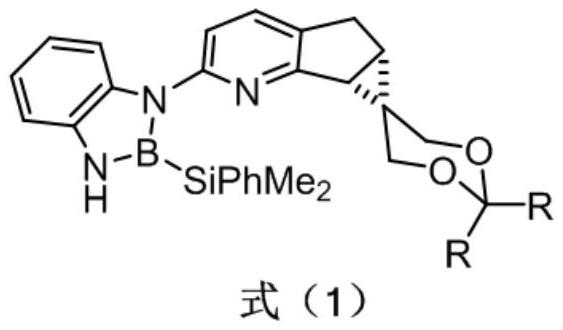
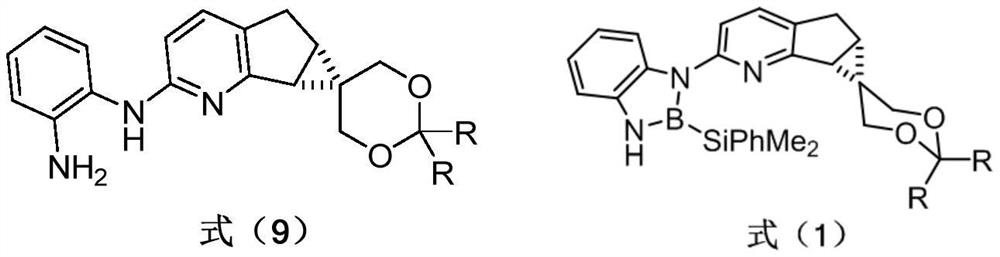
Chiral pyridine-derived N, B ligand, preparation method and application in iridium-catalyzed asymmetric boronation reaction
A chiral and pyridine technology, which is applied in the application field of iridium-catalyzed asymmetric borylation reaction, can solve the problems of contradiction between activity and selectivity, poor structure modifiability, difficult modular synthesis, etc., and achieve excellent enantioselectivity, The cost of raw materials is low, and the effect of synthesis is easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
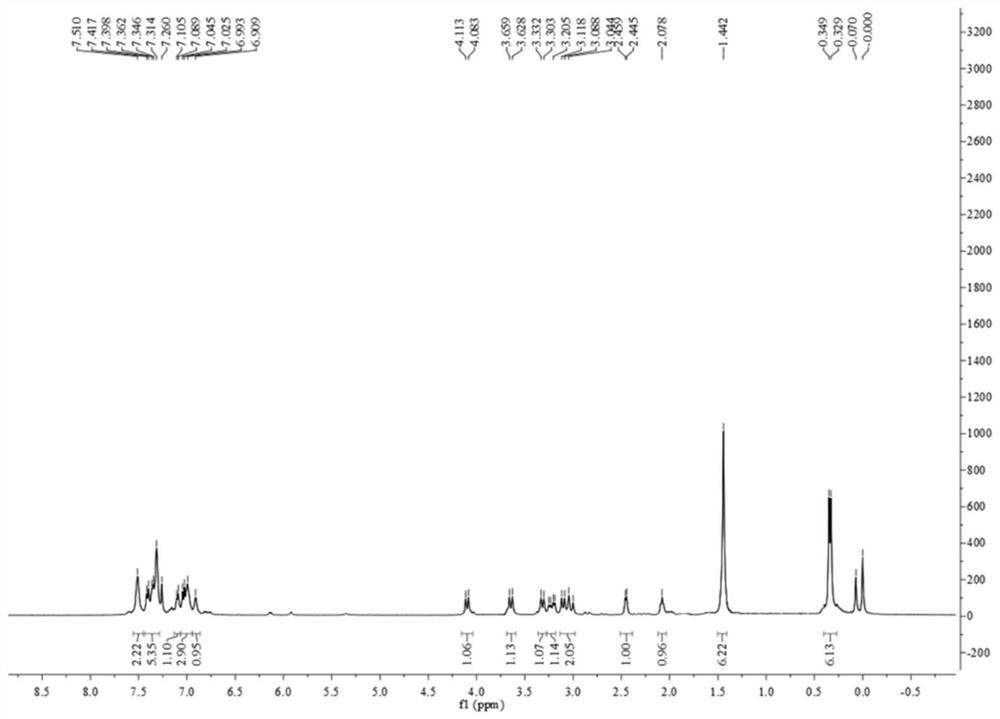
[0081] Synthesis of chiral pyridine-derived N,B ligands:
[0082] 1.1)
[0083]
[0084]In a 250mL single-necked bottle, add bromocyclopentenone (17.71g, 110mmol), diethyl malonate (25.05mL, 165mmol), tetrahexylammonium bromide (4.78g, 11mmol), potassium carbonate (91.21g , 660mmol), and 60mL of 1,2-dichloroethane, heated to reflux at 90°C for 8 hours. Aftertreatment: filter the reaction solution with a Buchner funnel, rinse the filter cake with dichloromethane, collect the filtrate, concentrate, separate and purify by column chromatography to obtain the racemic ketone (colorless oily liquid, 86% yield) shown in formula (2) .
[0085] 1.2)
[0086]
[0087] The racemic ketone represented by formula (2) (9.6g, 40mmol), (R)-tert-butylsulfinamide (5.33g, 44mmol), tetraethyl titanate (18.25g, 80mmol), 40mL without Mix water and 1,2-dichloroethane, and heat to reflux at 90°C for 12 hours. Post-reaction treatment: Quench the reaction with saturated ammonium chloride solut...
Embodiment 2
[0111] 1.1) In a 10mL single-necked bottle, add bromocyclopentenone (1mmol), diethyl malonate (1.0mmol), tetrahexylammonium bromide (0.15mmol), potassium carbonate (4mmol), and 2mL of 1, 2-dichloroethane, heated to reflux at 84°C for 10 hours. Aftertreatment: filter the reaction solution with a Buchner funnel, rinse the filter cake with dichloromethane, collect the filtrate, concentrate, separate and purify by column chromatography to obtain the racemic ketone (colorless oily liquid, 86% yield) shown in formula (2) .
[0112] 1.2) Mix the racemic ketone (1mmol) represented by formula (2), (R)-tert-butylsulfinamide (1.2mmol), tetraethyl titanate (3mmol), 2mL of anhydrous 1,2-bis Mix ethyl chloride and heat to reflux at 80°C for 18 hours. Post-reaction treatment: Quench the reaction with saturated ammonium chloride solution at 0°C, filter with suction, collect the filtrate, extract the aqueous phase with dichloromethane three times, combine the organic phase, back-extract with...
Embodiment 3
[0121] 1.1) In a 10mL single-necked bottle, add bromocyclopentenone (1mmol), diethyl malonate (1.5mmol), tetrahexylammonium bromide (0.2mmol), potassium carbonate (5mmol), and 2mL of 1, 2-dichloroethane, heated to reflux at 80°C for 12 hours. Aftertreatment: filter the reaction solution with a Buchner funnel, rinse the filter cake with dichloromethane, collect the filtrate, concentrate, separate and purify by column chromatography to obtain the racemic ketone (colorless oily liquid, 86% yield) shown in formula (2) .
[0122] 1.2) Mix the racemic ketone (1mmol) represented by formula (2), (R)-tert-butylsulfinamide (1.5mmol), tetraethyl titanate (2.5mmol), 2mL of anhydrous 1,2- Dichloroethane was mixed and heated to reflux at 85°C for 15 hours. Post-reaction treatment: Quench the reaction with saturated ammonium chloride solution at 0°C, filter with suction, collect the filtrate, extract the aqueous phase with dichloromethane three times, combine the organic phase, back-extrac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com