Sustained delivery of angiopoetin-like 3 polypeptides
A delivery system, C3-C6 technology, applied in the field of sustained release of therapeutic agents, can solve problems such as difficult controllability and difficult drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
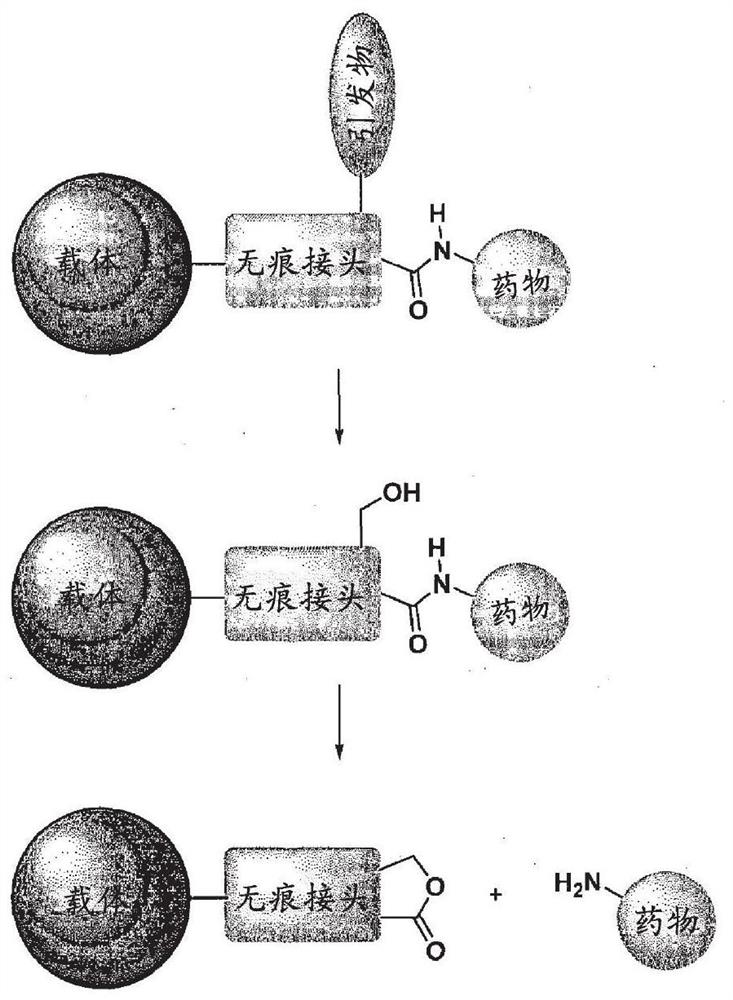
[0659] Seamless joint
[0660] This example describes the synthesis of a number of traceless linkers capable of conjugating both amine-containing drugs and carriers.
[0661]
[0662]
[0663] Synthesis of seamless joints
[0664] Common intermediate: L-INT-1c
[0665]
[0666] L-INT-1c.1-(Boc)-2-(hydroxymethyl)-4-(N-Cbz-β-alanyl)piperazine
[0667]
[0668] A 250-mL flask was charged with tert-butyl 2-(hydroxymethyl)piperazine-1-carboxylate SM-2 (5.81 g, 26.9 mmol), 3-(((benzyloxy)carbonyl)amino)propane Acid SM-1 (5 g, 22.4 mmol) and acetonitrile (100 mL). To this suspension was added triethylamine (9.37 mL, 67.2 mmol), HOBt (0.686 g, 4.48 mmol) and EDC.HCl (6.44 g, 33.6 mmol) in sequence. The reaction mixture was stirred at room temperature for 15 h. After this time, the mixture was diluted with water and extracted three times with ethyl acetate. The combined organic layers were washed sequentially with 1M HCl solution, NaHCO 3 Wash with saturated solution...
example 2
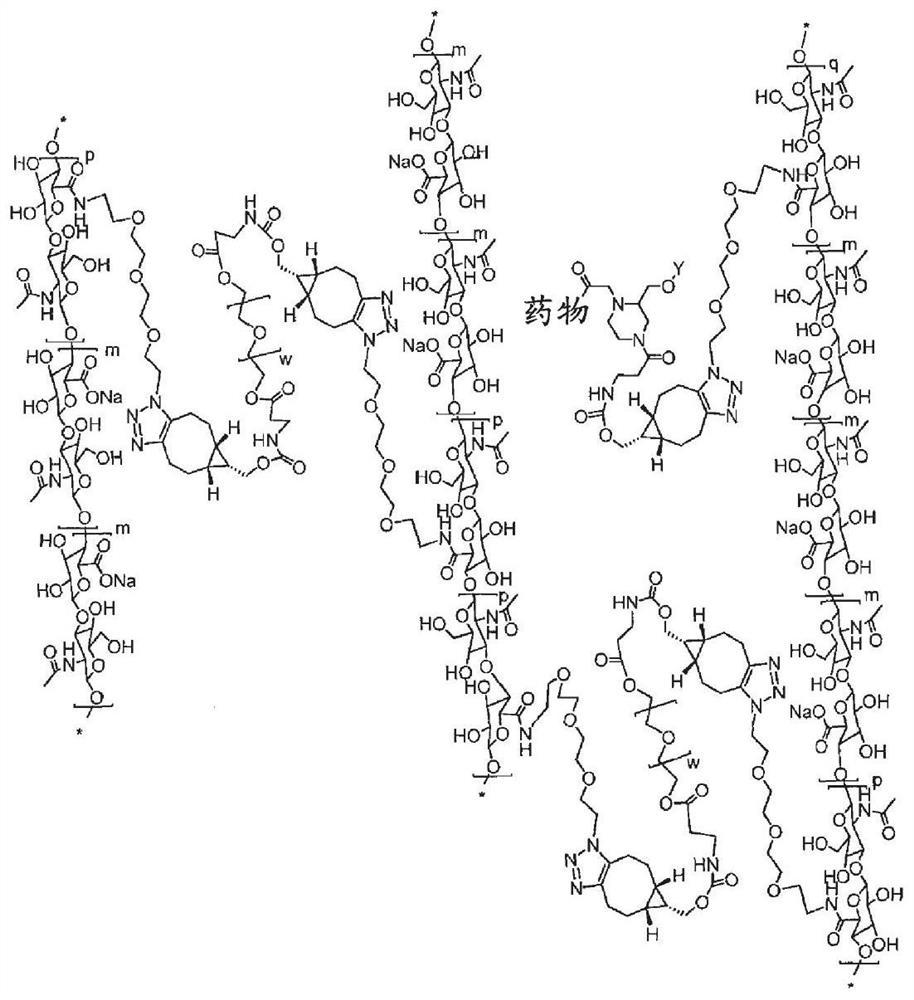
[0775] Adducts of traceless linkers with bioactive moieties
[0776] This example describes the synthesis of a number of traceless linker-drug adducts that are also capable of being conjugated to a carrier. D1 as depicted below comprises an ANGPTL3 polypeptide (SEQ ID NO: 19) comprising amino acid residues 242-460 of reference SEQ ID NO: 1 and a K423Q substitution.
[0777]
[0778]
[0779] Synthesis of adducts
[0780] Acylation of bioactive moieties with traceless linkers:
[0781] L2-NHS.2-(Acetoxymethyl)-4-(N-(((1R',8'S,9's)-bicyclo[6.1.0]non-4'-yne-9'- Base) methoxycarbonyl) -β-alanyl) -1-piperazine acetic acid N-hydroxysuccinimidyl ester
[0782]
[0783] L2 (16 mg, 0.031 mmol) was dissolved in dimethyl sulfoxide (0.818 mL) to achieve a concentration of 38 mM. Triethylamine (3.46 μL, 0.025 mmol) was added followed by N,N′-disuccinimidyl carbonate (11.94 mg, 0.047 mmol) and the resulting clear solution was stirred at room temperature under argon for 1 h . ...
example 3
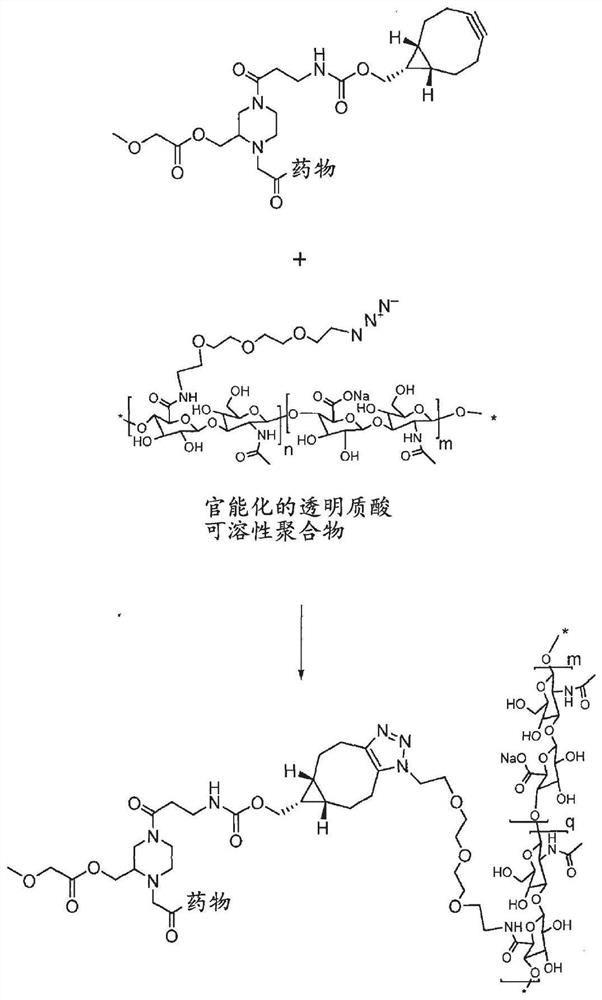
[0800] Functionalization of hyaluronic acid
[0801] This example describes the synthesis of functionalized hyaluronic acid, which can be a carrier itself, or can also be reacted with a crosslinking moiety to form a hydrogel.
[0802] Synthesis of hyaluronic acid intermediate [HA-N3]:
[0803]
[0804] Hyaluronic acid sodium salt is a linear polymer consisting of repeating dimer units of glucuronic acid and N-acetylgalactosamine, wherein the repeating unit has a molecular weight of 401.3Da. In this example, the number of moles of hyaluronic acid reported refers to the number of moles of repeating units, and the equivalents of reagents used to react with hyaluronic acid are reported relative to the number of moles of repeating units of hyaluronic acid. The average molecular weight of a polymer determines the average number of repeating units per polymer chain. Batches of hyaluronic acid sodium salt labeled as having a nominal average molecular weight of 200 kDa by the supp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com