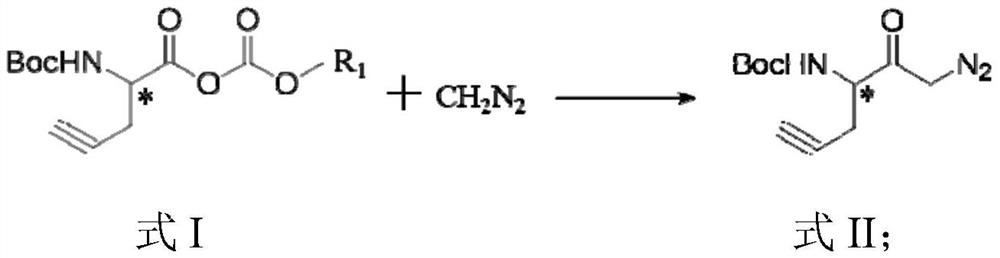Preparation method of chiral beta-(Boc-amino)-5-hexynoic acid
A hexynoic acid and amino technology, applied in the field of preparation of chiral β--5-hexynoic acid, can solve the problems of high production cost and complicated preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
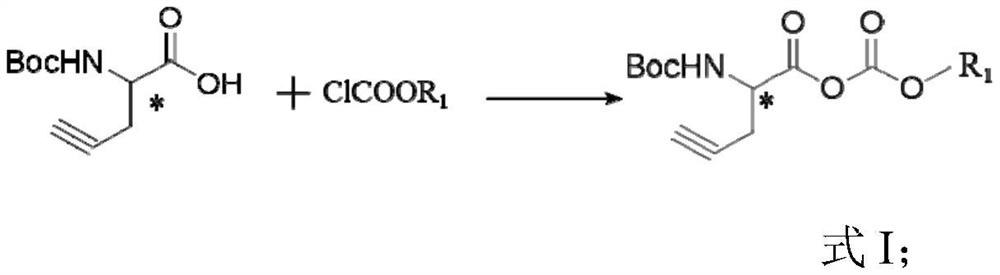
[0088]A method of preparation of (R) -β- (BOC-amino) -5-hexynone acid, specifically, including the steps of:
[0089](1)
[0090](R) -α- (BOC-amino) -4-pentanylic acid (1.5 g, 7.035 mmol) and triethylamine (0.93 g, 9.145 mmol) were added to 10 ml of tetrahydrofuran, under nitrogen protection, at 0 ° C Ethyl chloroformate (0.992 g, 9.145 mmol) was dripped, and after the dropwise addition was completed, 60 min was reacted at 0 ° C to obtain a product.Resulting productUnstable, no purification is required to directly carry out the next step.
[0091](2)
[0092]At -15 ° C, the isopropyl ether solution (38.69 mL) (38.69 mL) of a diazomethane (38.69 mL) drop gravy concentration of 1 mol / L was obtained from step (1), and under the condition of -15 ° C, the reaction was 20 min; heated to At 0 ° C, a acetic acid solution (21.12 g) of a 12% percentage of a mass of 10% was added dropwise, and the reaction was 30 min under 0 ° C.There is no need to purify directly into the next step.
[0093](3)
[0094]At -5...
Embodiment 2
[0099]A method of preparation of (S) -β- (BOC-amino) -5-hexynum acid, specifically, including the steps of:
[0100](1)
[0101](S) -α- (BOC-amino) -4-pentanylic acid (1.8 g, 8.441 mmol) and N-methylmorpholine (1.02 g, 10.13 mmol) were added to 10 ml of methyl tert-butyl ether, Under argon, Ethyl chloroformate (1.099 g, 10.13 mmol) was added dropwise to -5 ° C, and the reaction was added to 2 ° C for 55 min.Resulting productUnstable, no purification is required to directly carry out the next step.
[0102](2)
[0103]At -13 ° C, a methyl tert-butyl ether solution (63.91 mL) dropped to the reaction solution to the reaction liquid, and under the condition of -13 ° C, the reaction is obtained from the step (1). 15 min; heated to -3 ° C, a propionic acid solution (33.11 g) of 10% by weight of mass was added dropwise, and the reaction was 32 min under the condition of -3 ° C to obtain a productThere is no need to purify directly into the next step.
[0104](3)
[0105]At -3 ° C, the reaction solution obta...
Embodiment 3
[0109]A method of preparation of (R) -β- (BOC-amino) -5-hexynone acid, specifically, including the steps of:
[0110](1)
[0111](R) -α- (BOC-amino) -4-pentanylic acid (2 g, 9.38 mmol) and N, N-diisopropylethylamine (1.33 g, 10.32 mmol) were added to 10 ml of tetrahydrofuran, in nitrogen Under the protected, Ethyl chloroformate (1.272 g, 11.72 mmol) was dripped at -3 ° C, and after the dropwise addition was completed, the reaction was 10 min to obtain a product.Resulting productUnstable, no purification is required to directly carry out the next step.
[0112](2)
[0113]At -12 ° C, the isopropyl ether solution (52.11 mL) of aidazole concentration of 0.9 mol / L is obtained from the step (1), and the reaction is 18 min under the condition of -12 ° C; temperature rise By 5 ° C, a acetic acid solution (28.16 g) of 10% by weight of mass was added dropwise, and the reaction was 35 min under the conditions of 5 ° C.There is no need to purify directly into the next step.
[0114](3)
[0115]At 5 ° C, the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com