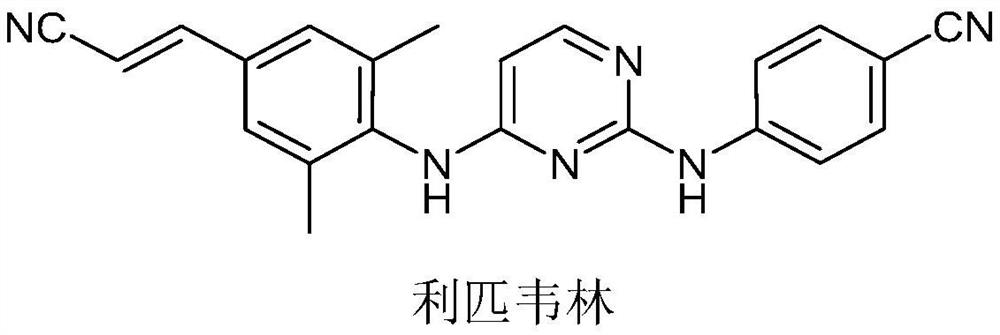
Intermediate for synthesizing rilpivirine, synthesis method thereof and synthesis method of rilpivirine
A technology of rilpivirine and its synthesis method, which is applied in the field of synthesizing rilpivirine intermediates and rilpivirine, and can solve problems such as reducing the overall yield of rilpivirine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
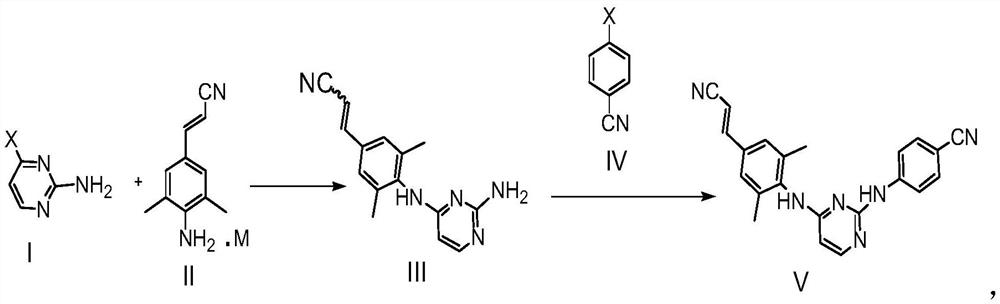
[0050] The embodiment of the present invention provides a synthesis method of 3-(4-(2-aminopyrimidin-4-yl)-3,5-dimethylphenyl)-acrylonitrile:
[0051] Refer to the synthesis path below:
[0052] Specific steps are as follows:
[0053] Put DMF (40ml) into the reaction flask, add the compound represented by formula I (14.3g, 110mmol) and the compound represented by formula II-1 (20.9g, 100mmol), under nitrogen protection, stir, and heat up to 90°C-95°C. This temperature was maintained for 3 hours. After the reaction was completed, the temperature was lowered to room temperature 20±5° C., and 120 ml of 10% potassium carbonate aqueous solution was added dropwise. A solid precipitated, filtered and washed with water. Filter until no filtrate drips, scrape off the filter cake, reflux with 30ml acetonitrile for beating for 1 hour, cool to room temperature 20±5°C, filter, rinse with an appropriate amount of acetonitrile, and dry at 50°C to obtain the intermediate shown in formula...
Embodiment 2
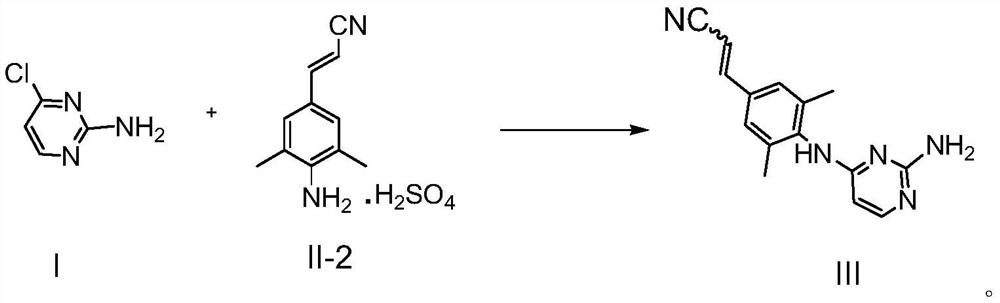
[0057] The embodiment of the present invention provides a synthesis method of 3-(4-(2-aminopyrimidin-4-yl)-3,5-dimethylphenyl)-acrylonitrile:
[0058] Refer to the synthesis path below:
[0059]
[0060] Put DMF (50ml) into the reaction flask, add the compound represented by formula I (14.3g, 110mmol) and the compound represented by formula II-2 (27g, 100mmol), under nitrogen protection, stir, and heat up to 90°C-95°C. This temperature was maintained for 3 hours. After the reaction was completed, the temperature was lowered to room temperature 20±5° C., and 150 ml of 10% potassium carbonate aqueous solution was added dropwise. A solid precipitated, filtered and washed with water. Filter until no filtrate drips, scrape off the filter cake, reflux with 30ml acetonitrile for beating for 1 hour, cool to room temperature 20±5°C, filter, rinse with an appropriate amount of acetonitrile, and dry at 50°C to obtain the intermediate shown in formula III (19.9g , 75%, purity 97.5%,...
Embodiment 3
[0064] The embodiment of the present invention provides a synthesis method of 3-(4-(2-aminopyrimidin-4-yl)-3,5-dimethylphenyl)-acrylonitrile:
[0065] Refer to the synthesis path below:
[0066]
[0067] Put DMF (70ml) into the reaction flask, add the compound represented by formula I (14.3g, 110mmol) and the compound represented by formula II-3 (34.4g, 100mmol), under nitrogen protection, stir, and heat up to 90°C-95°C. This temperature was maintained for 3 hours. After the reaction was completed, the temperature was lowered to room temperature 20±5° C., and 200 ml of 10% potassium carbonate aqueous solution was added dropwise. A solid precipitated, filtered and washed with water. Filter until no filtrate drips, scrape off the filter cake, reflux with 30ml acetonitrile for beating for 1 hour, cool to room temperature 20±5°C, filter, rinse with an appropriate amount of acetonitrile, and dry at 50°C to obtain the intermediate shown in formula III (22.5g , 85%, purity 98.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com