Application of BPTES in preparation of medicine for preventing or treating anthracnose
An anthracnose, the technology of use, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 BPTES can inhibit macrophage death caused by anthrax toxin
[0040] J774A.1 mouse mononuclear macrophages were purchased from ATCC, DMEM medium + 10% FBS + non-essential amino acids + penicillin + streptomycin, at 37°C, 5% CO 2cultivated in the environment. BPTES (HY-12683) was purchased from MedChem Express (MCE), and dissolved in DMSO to prepare the corresponding concentration. Cells were divided into control group (Ctrl), DMSO group, BPTES 2μM group, BPTES 5μM group, BPTES 10μM group. Among them, the DMSO group, BPTES 2 μM group, BPTES 5 μM group, and BPTES 10 μM group were treated with 2 μg / mL LF+PA for 3 h, and the BPTES 2 μM group, BPTES 5 μM group, and BPTES 10 μM group were given corresponding concentrations of BPTES. The cell viability was tested using the lactate dehydrogenase (LDH) release method with the LDH detection kit from Promega.
[0041] The data were processed using GraphPad 6.0 software, expressed as mean ± standard error (means ± SEM),...
Embodiment 2
[0043] Example 2 BPTES can prevent the death of mice caused by anthrax toxin
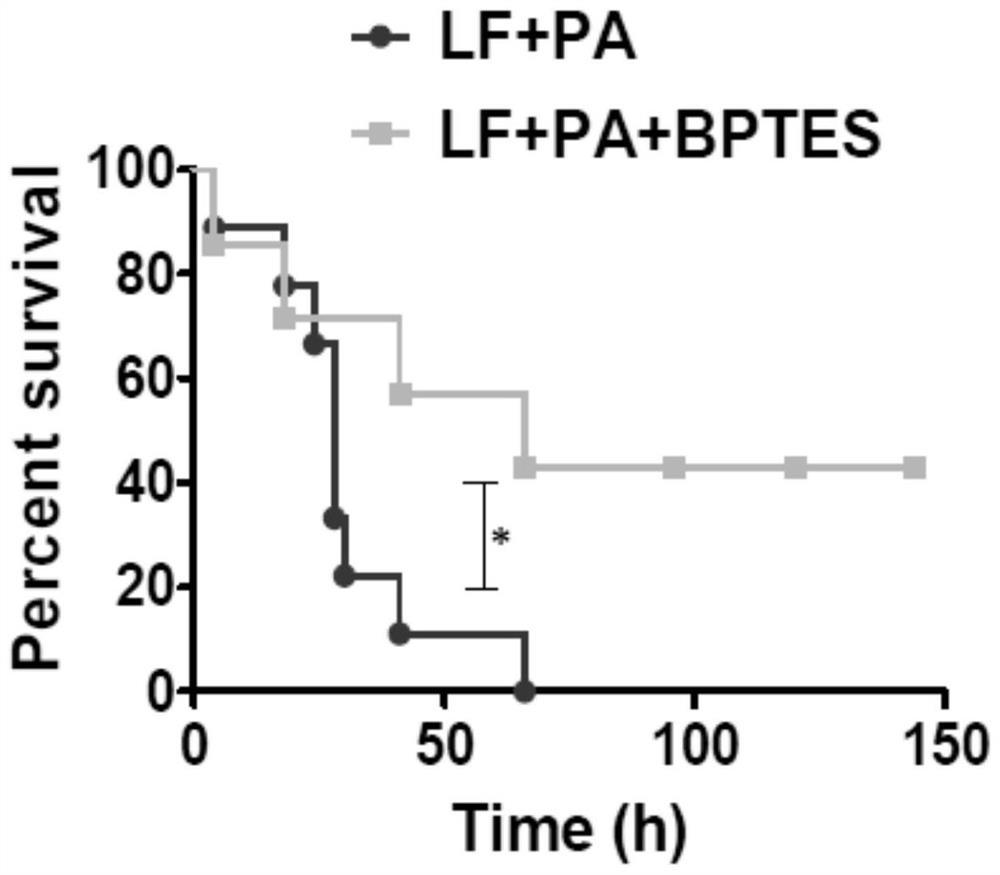
[0044] 4-week-old female Balb / c mice were from the Experimental Animal Center of Xiamen University, and were divided into a solvent group (LF+PA) and a BPTES-administered group (LF+PA+BPTES), with N=9 in each group. The solvent group was intraperitoneally injected with vehicle (DMSO), the drug group was injected with 1 mg / kg BPTES intraperitoneally, and the solvent group and the drug group were intravenously injected with 500 mg / kg LF+PA respectively. Mice were measured up to 150 hours alive. The survival rate of mice was expressed by Kaplan-Meyer survival curve, and the log-rank (Mantel-Cox) test was performed. *p<0.05, ***p<0.001.
[0045] The result is as figure 2 As shown, 50% of the BPTES administration group survived, and all of the solvent group died, indicating that BPTES can prevent the death of mice caused by anthrax lethal toxin.
Embodiment 3
[0046] Example 3 BPTES can reduce the inflammation caused by anthrax toxin
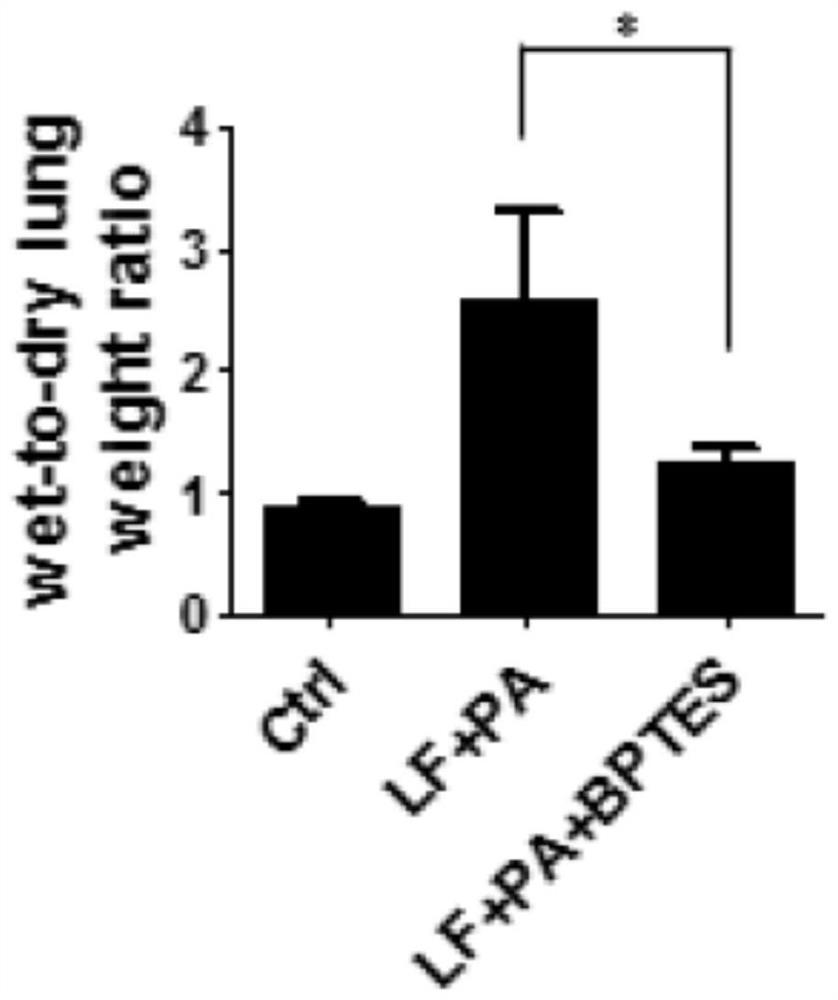
[0047] 4-week-old female Balb / c mice were divided into control group (Ctrl), solvent group (LF+PA) and BPTES administration group (LF+PA+BPTES), each group N=4. The control group (Ctrl) was intraperitoneally injected with PBS solution, and the administration methods of the solvent group (LF+PA) and the BPTES administration group (LF+PA+BPTES) were referred to Example 2. Administration time 30min.
[0048] The mice in each group were killed, and the lungs of the mice were taken, and the wet weight and dry weight of the lungs were measured, and the ratio of the wet weight to the dry weight of the lungs was calculated by dividing the wet weight by the dry weight. The results were as follows: image 3 As shown, it was found that the pulmonary edema of mice administered with BPTES was significantly reduced.
[0049] The pleural effusion of mice in each group was drained and quantified, and the results we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com