Novel coronavirus nucleic acid quantitative detection kit based on micro-droplet digital analysis
A detection kit, a technology for coronaviruses, applied in the determination/inspection of microorganisms, resistance to vector-borne diseases, biochemical equipment and methods, etc., can solve the loss of samples, the need for repeated detection, the complexity of primer and detection probe design And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
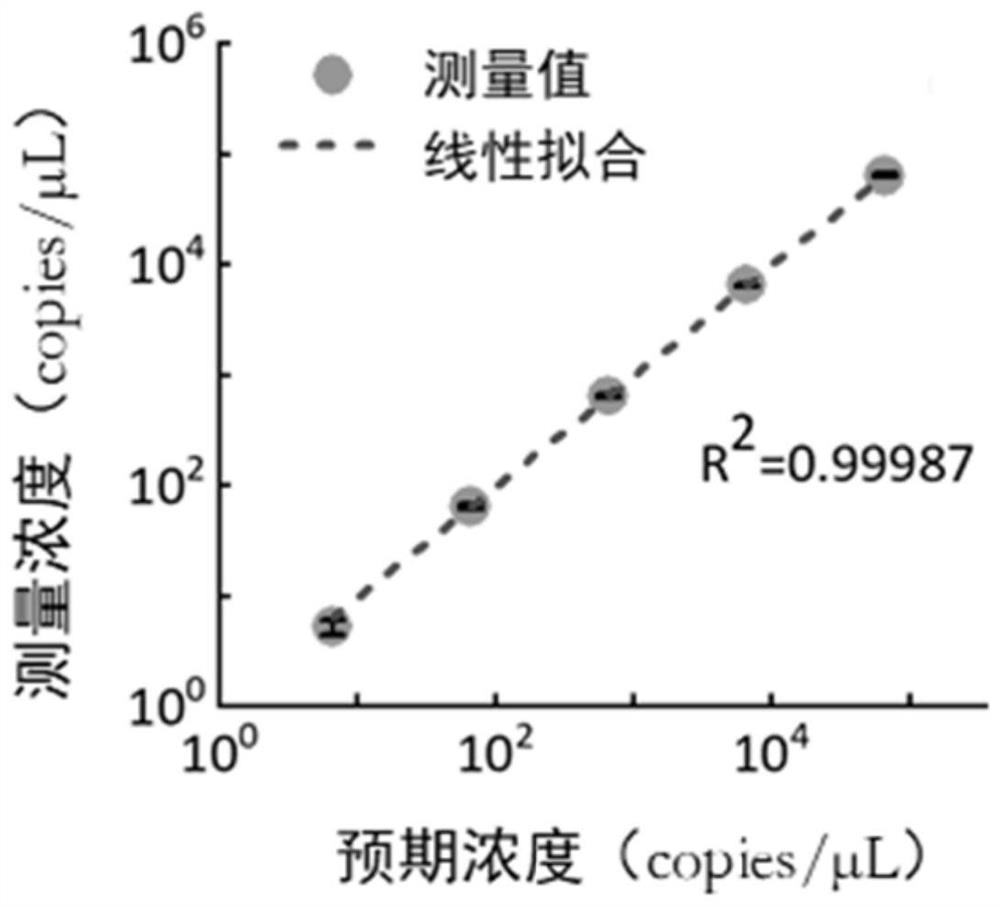
[0036] Embodiment 1, Absolute Quantitative Detection SARS-CoV-2RNA Quantitative Reference Substance
[0037] A novel coronavirus nucleic acid quantitative detection kit based on micro-droplet digital analysis, characterized in that it includes O-crRNA targeting SARS-CoV-2 RNA ORF1ab gene 6513-6537nt and targeting SARS- N-crRNA of N gene 29214-29238nt of CoV-2 RNA, Cas13a protein, single-stranded RNA reporter probe and reaction buffer.
[0038] (1) RNA sample preparation: The SARS-CoV-2 RNA quantitative reference product used in this experiment was purchased from the National Institute of Metrology, China. The standard product contains three characteristic genes of SARS-CoV-2: full-length N gene, full-length Fragments (13201-15600 nt) of the long E gene and the orf1ab gene, whose nominal RNA concentrations were determined by droplet digital PCR.
[0039] (2) Reaction system preparation: first prepare 9 μL reaction premix, which contains 1 μL reaction buffer (Tris-HCl, MgCl 2,...
Embodiment 2
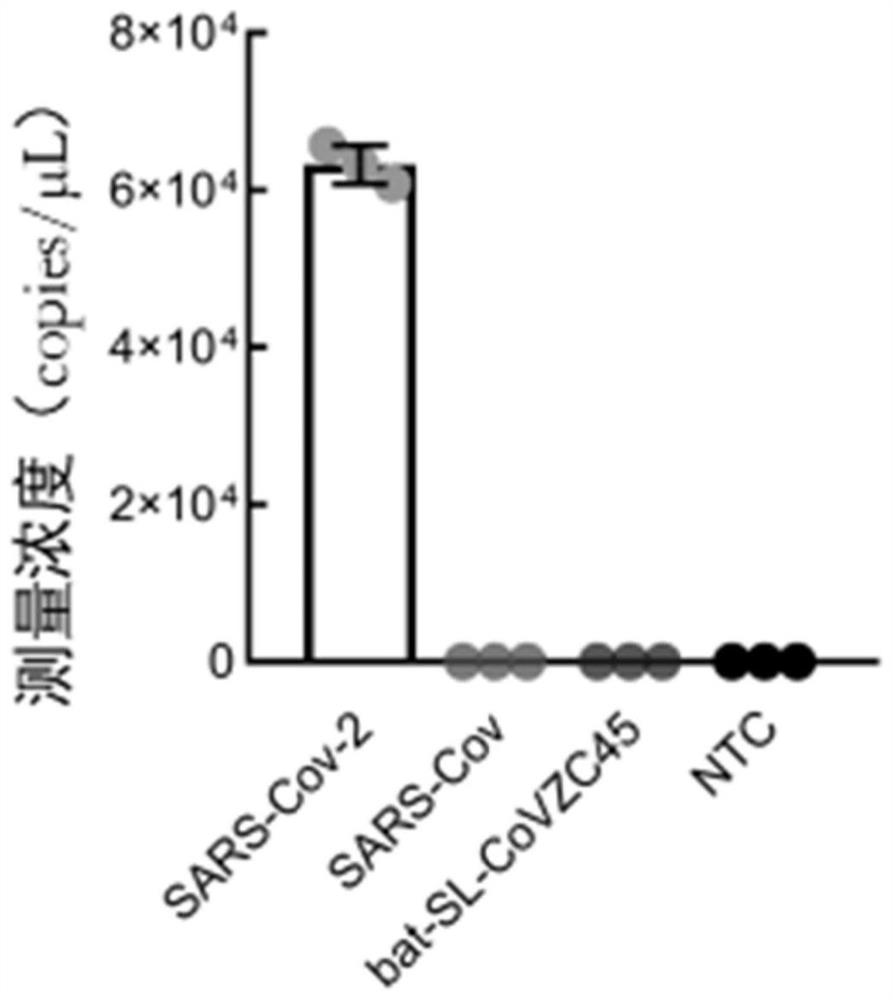
[0044] Embodiment 2, detection specificity test
[0045] A novel coronavirus nucleic acid quantitative detection kit based on micro-droplet digital analysis, characterized in that it includes O-crRNA targeting SARS-CoV-2 RNA ORF1ab gene 6513-6537nt and targeting SARS- N-crRNA of N gene 29214-29238nt of CoV-2 RNA, Cas13a protein, single-stranded RNA reporter probe and reaction buffer.
[0046] (1) In vitro transcription of N genes corresponding to three kinds of coronaviruses used to test N-crRNA specificity - SARS-CoV-2, SARS virus (SARS-CoV) and bat SARS-like coronavirus (bat-SL-CoVZC45) The corresponding NCBI accession numbers of the products are NC_045512, NC_004718 and MG772933, respectively. Before the test, the above in vitro transcription products were adjusted to the same concentration level using a UV spectrophotometer.
[0047] (2) Prepare a reaction system whose components mainly include 20nM LbuCas13a protein, 100nM N-crRNA, 300nMFQ 5U RNA fluorescent reporter pr...
Embodiment 3
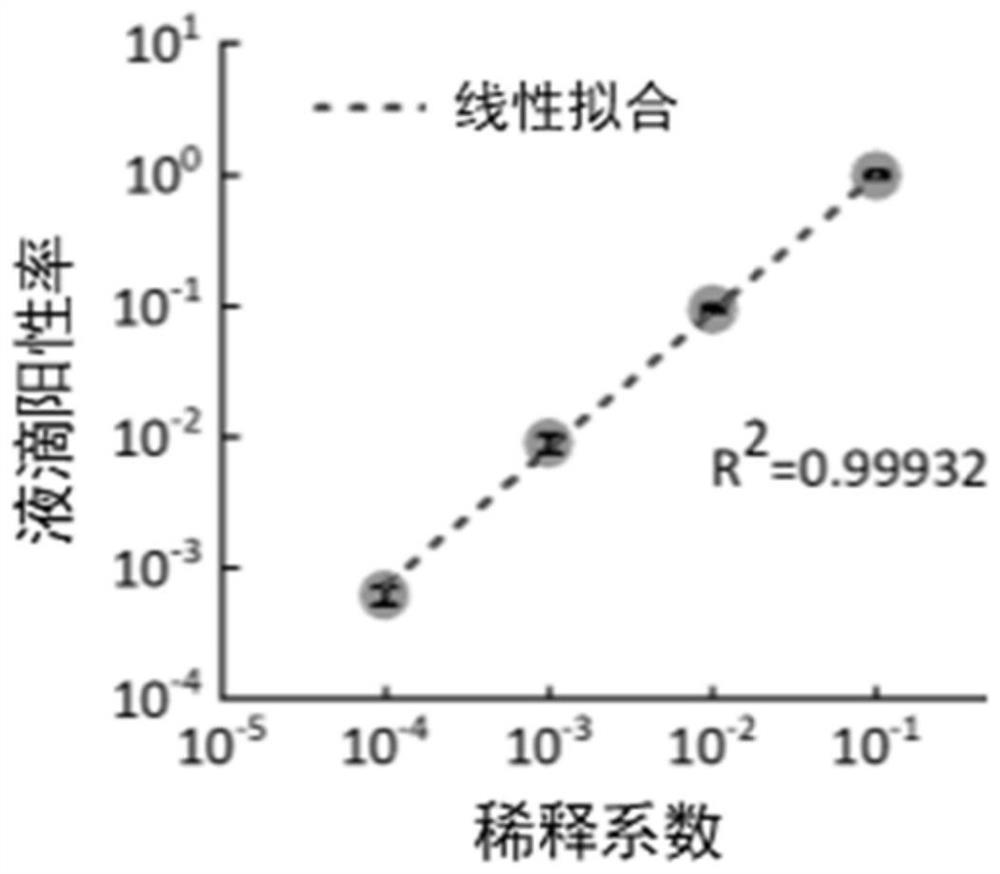
[0055] Example 3, Quantitative detection of full-length SARS-CoV-2 RNA
[0056] A novel coronavirus nucleic acid quantitative detection kit based on micro-droplet digital analysis, characterized in that it includes O-crRNA targeting SARS-CoV-2 RNA ORF1ab gene 6513-6537nt and targeting SARS- N-crRNA of N gene 29214-29238nt of CoV-2 RNA, Cas13a protein, single-stranded RNA reporter probe and reaction buffer.
[0057] (1) Sample preparation: The full-length RNA samples of the SARS-CoV-2 genome were provided by the Hubei Provincial Center for Disease Control and Prevention for scientific research purposes, and were serially diluted 10 times with RNase-free water before testing.
[0058] (2) Prepare a reaction system whose components mainly include 20nM LbuCas13a protein, 100nM N-crRNA, 300nMFQ 5U RNA fluorescent reporter probe (FAM-UUUUU-BHQ1), 1× reaction buffer, wherein the reaction buffer contains 10mMTris-HCl , 1.5mM MgCl 2 , 50mM KCl, solution pH 8.9.
[0059] (3) Mix the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com