Adeno-associated virus vector delivery of muscle specific micro-dystrophin to treat muscular dystrophy
A small technology for muscular dystrophy, applied in the direction of muscle protein, muscle system disease, virus/phage, etc., can solve problems such as limiting muscle regeneration, muscle fiber degeneration, necrosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
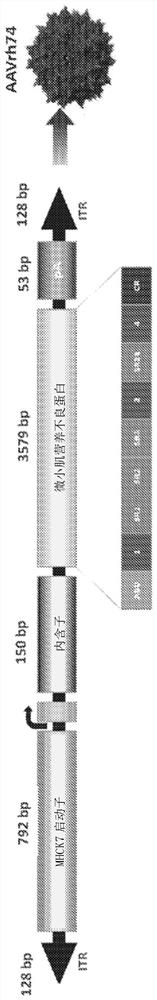
[0179] A) Generation of AAVrh74.MHCK7.mini-dystrophin constructs
[0180] The AAVrh74.MHCK7.microdystrophin particle contains a human microdystrophin cDNA expression cassette flanked by AAV2 inverted terminal repeats (ITRs) (see figure 1 ). The mini-dystrophin construct is characterized by an in-frame rod domain deletion (R4-R23), while hinges 1, 2 and 4 and the cysteine-rich domain still give rise to a 138 kDa protein. Expression of microdystrophin (3579bp) is directed by the MHCK7 promoter (792bp). The plasmid was constructed by removing the MCK promoter from the rAAV.MCK.microdystrophin plasmid and inserting the MHCK7 promoter. After the core promoter, a 53bp endogenous mouse MCK exon 1 (untranslated) is provided for efficient transcription initiation, followed by the SV40 late 16S / 19S splicing signal (150bp) and a small 5'UTR (61bp ). Introns and 5'UTR were derived from plasmid pCMVβ (Clontech). The mini-dystrophin cassette has a consensus sequence Kozak preceding the...
Embodiment 2
[0187] Systemic gene delivery clinical trial for Duchenne muscular dystrophy
[0188] This is a single dose controlled trial using rAAVrh74.MHCK7.microdystrophin of SEQ ID NO:3, nucleotides 55-5021, in DMD subjects. Cohort A will include six subjects aged 3 months to 3 years and Cohort B will include six subjects aged 4 to 7 years. All subjects will receive intravenous microdystrophin carrier (2 x 10 14 vg / kg, 10mL / kg) injection. rAAVrh74.MHCK7.microdystrophin in the presence of 20mM Tris (pH8.0), 1mM magnesium chloride (MgCl 2 ), 200mM sodium chloride (NaCl) and 0.001% poloxamer 188 buffer.
[0189] In this study, rAAVrh74.MHCK7.microdystrophin was infused via a peripheral arm vein, allowing access to all muscles in the body. Six DMD subjects aged 3 months to 3 years were enrolled in Cohort A, and six DMD subjects aged 4 to 7 years were enrolled in Cohort B. All subjects received intravenous microdystrophin carrier (2 × 10 14 vg / kg, 10mL / kg) injection. The capsid-encap...
Embodiment 3
[0256] A randomized double-blind placebo-controlled phase I / IIa clinical trial of systemic gene delivery
[0257] This is a randomized double-blind single-dose trial with rAAVrh74.MHCK7.microdystrophin in DMD subjects. The study included twenty-four subjects aged 4 to 7 years. Subjects were randomly assigned to treatment or placebo at enrollment. Twelve subjects received intravenous rAAVrh74.MHCK7.mini-dystrophin vector (2×10 14 vg / kg, approximately 10 mL / kg), while another twelve subjects will receive 10 mL / kg placebo (Lactated Ringer's solution). Placebo subjects will continue treatment in the same manner as the 12 previously treated subjects one year after the last treated subject is dosed. Subjects received an infusion of rAAV with microdystrophin or lactated Ringer's solution over approximately 1 hour. Needle muscle biopsies were performed on the gastrocnemius muscle before and after treatment (90 days).
[0258] The primary objective of the study was to evaluate the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com