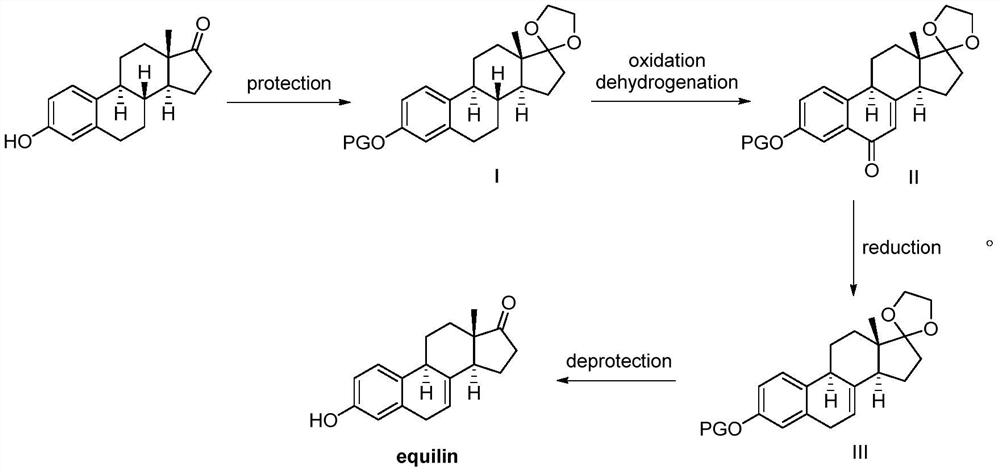
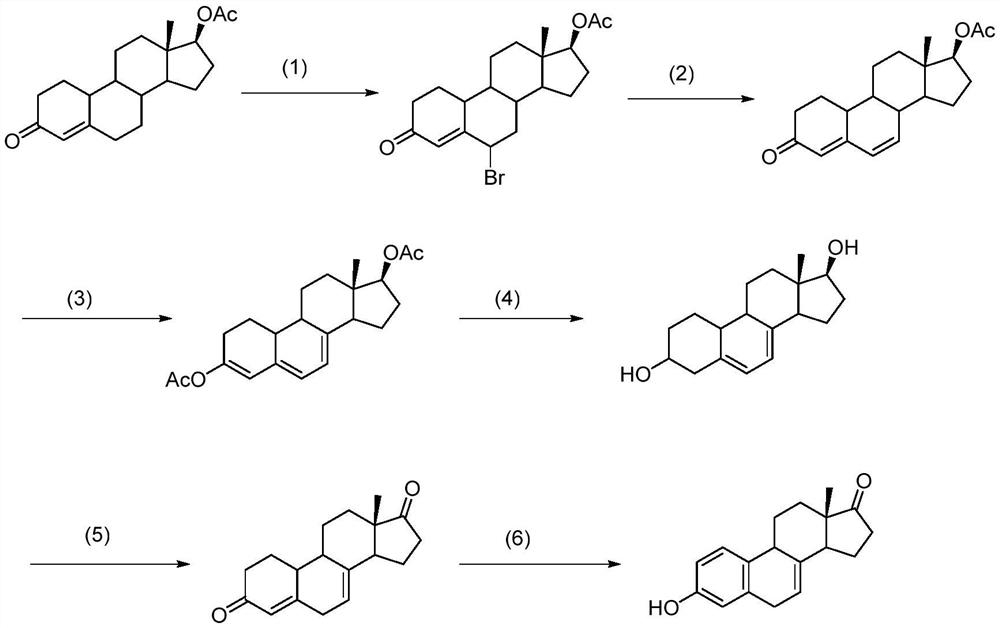
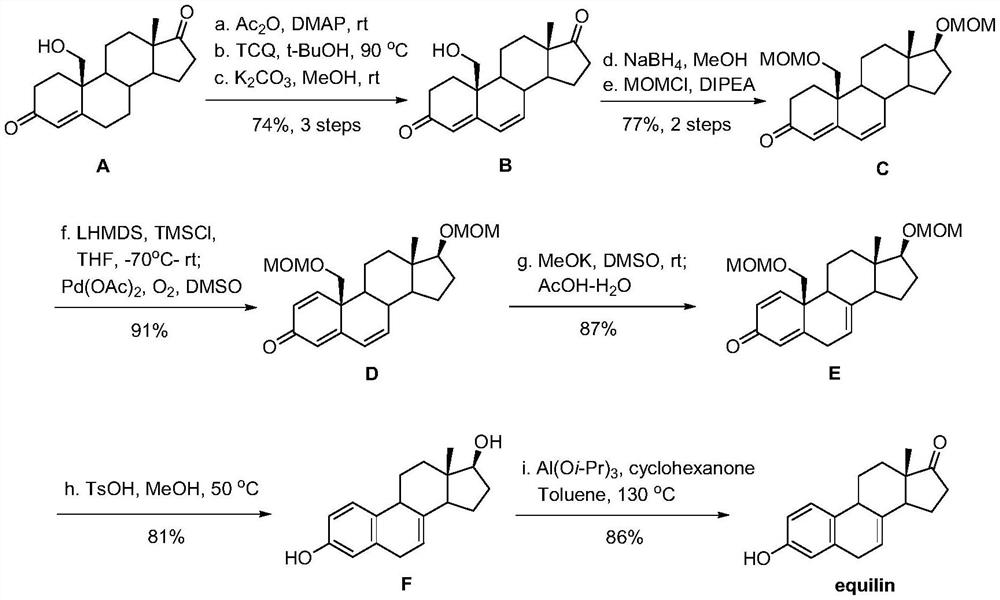
A kind of method of synthesizing equilenone
A technology for the synthesis of equilin and its synthesis method, which is applied in the field of synthesis of steroid drug equilin, and can solve the problems of environmental pollution, high equipment requirements, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Synthesis of intermediate I (C3 acetyl protection; C17 ethylene glycol ketal protection):
[0025] Add acetic anhydride (150mmol, 15.3g) and 4-dimethylaminopyridine (3mmol, 0.367g) successively to estrone (30mmol, 8.1g), stir at room temperature for 15 minutes, thin layer chromatography shows that estrone has been converted completely. Concentrate and add dichloromethane (20mL) to dilute, add saturated NaHCO 3 Solution to system is neutral. After liquid separation, the organic phase was washed with water, saturated brine, anhydrous Na 2 SO 4 After drying, filter through silica gel and suck dry (recover the solvent) to obtain a white solid. Dissolve the white solid in anhydrous benzene (30 mL), add ethylene glycol (60 mmol, 3.72 g) and p-toluenesulfonic acid (3 mmol, 0.516 g), heat to reflux for 5 hours, and cool to room temperature. saturated Na 2 CO 3 solution, washed with water, and then washed with saturated brine, combined the organic phases, and d...
Embodiment 2
[0027] Use tert-butyryl, benzoyl, CBZ (benzyloxycarbonyl, O=C-OCH 2 Ph), BOC (tert-butoxycarbonyl) and p-toluenesulfonyl anhydride instead of acetic anhydride, the others are the same as in Example 1, and the yields of compound I are 75%, 70%, 78%, 72%, and 76%, respectively.
Embodiment 3
[0029] Acetyl, tert-butyryl, benzoyl, CBZ (benzyloxycarbonyl, O=C-OCH 2 Ph), BOC (tert-butoxycarbonyl) and p-toluenesulfonyl chloride instead of acetic anhydride, others are the same as in Example 1, and the yield of compound I is respectively 80%, 77%, 75%, 79%, 78%, 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com