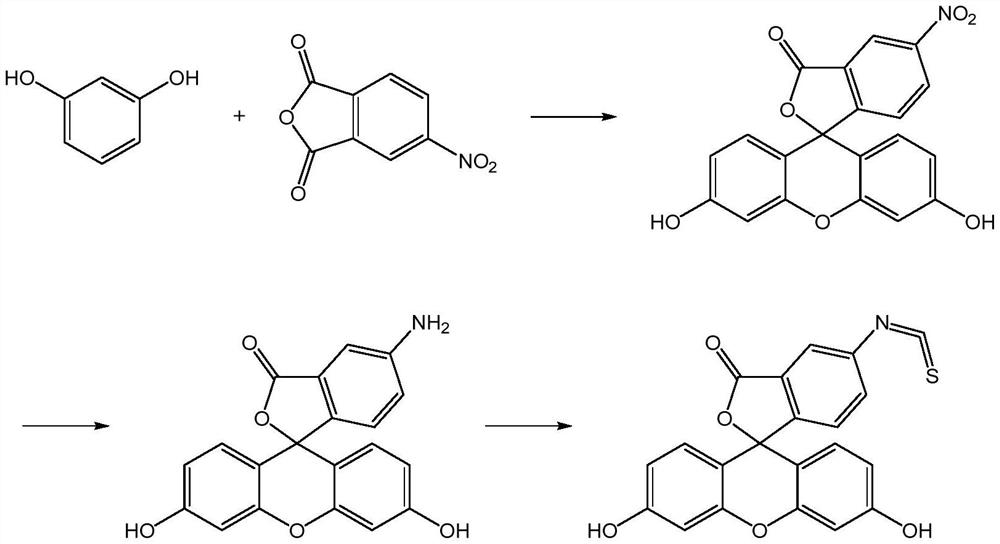
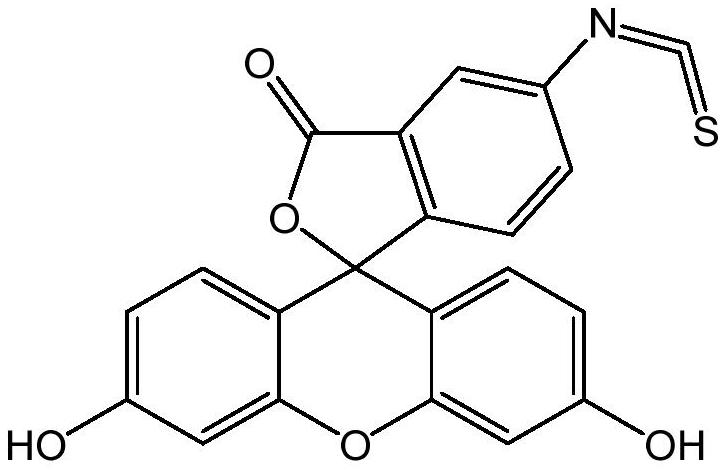
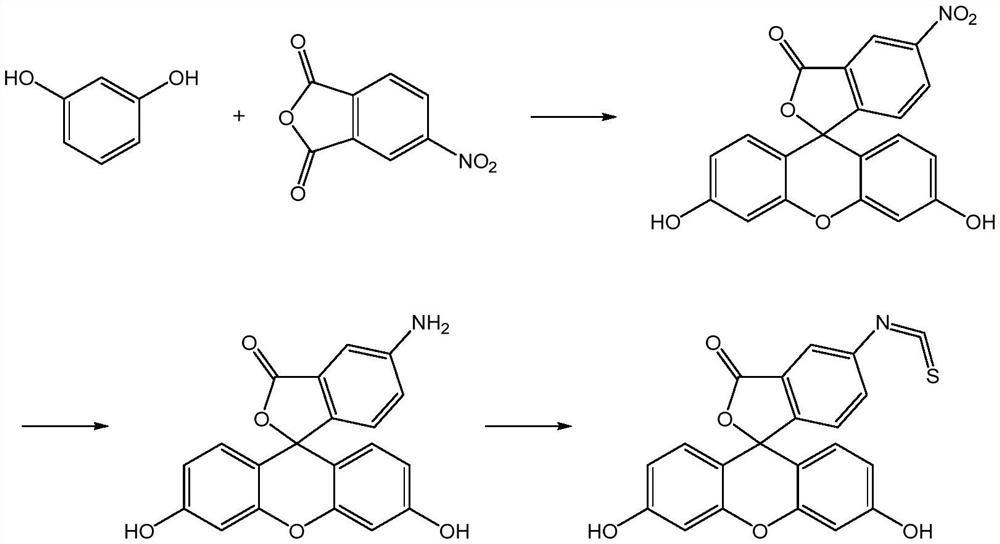
Preparation method of fluorescein probe with specific selectivity
A selectivity and fluorescein technology, applied in the field of preparation of fluorescein probes, can solve problems affecting the conversion rate and yield of 5-fluorescein isothiocyanate, achieve mild conditions, easy operation, improve conversion rate and Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The specific and selective fluorescein probe 5-fluorescein isothiocyanate is prepared according to the preparation method of the present application:
[0042](1) under the continuous stirring of 40rpm, the 4-nitrophthalic anhydride of 100g, the resorcinol of 118g, the nitrocyclohexane of 55g and the anhydrous cobalt chloride of 20g are mixed, Heat and melt at 125°C, add 100g of zinc chloride to it, react at 145°C for 4 hours, then add 1000mL of hydrochloric acid with a concentration of 0.75mol / L, heat and reflux for 40 minutes, filter, and filter the cake with 55°C Washed with water until neutral, dried at 85° C. for 2.5 hours to obtain 192.59 g of product (which contained 91.52% of 5-nitrofluorescein and 2.37% of 6-nitrofluorescein);
[0043] (2) Na 2 S and Na 2 SO 3 Dissolve in water to prepare reducing solutions with concentrations of 0.80 g / mL and 0.15 g / mL respectively, mix the product of step (1) with 1160 mL of the reducing solution, react at 75 ° C for 8.5 ho...
Embodiment 2
[0046] The specific and selective fluorescein probe 5-fluorescein isothiocyanate is prepared according to the preparation method of the present application:
[0047] (1) under the continuous stirring of 50rpm, the 4-nitrophthalic anhydride of 100g, the resorcinol of 120g, the nitrocyclohexane of 52g and the anhydrous cobalt chloride of 27g are mixed, Heat and melt at 115°C, add 100g of zinc chloride to it, react at 135°C for 5 hours, then add 1200mL of hydrochloric acid with a concentration of 0.75mol / L, heat and reflux for 45 minutes, filter, and filter the cake with 50°C Washed with water to neutrality, dried at 80° C. for 2 hours to obtain 193.45 g of product (which contained 92.38% of 5-nitrofluorescein and 2.15% of 6-nitrofluorescein);
[0048] (2) Na 2 S and Na 2 SO 3 Dissolve in water to prepare reducing solutions with concentrations of 0.60 g / mL and 0.20 g / mL respectively, mix the product obtained in step (1) with 1350 mL of the reducing solution, react at 80 ° C fo...
Embodiment 3
[0051] The specific and selective fluorescein probe 5-fluorescein isothiocyanate is prepared according to the preparation method of the present application:
[0052] (1) under the continuous stirring of 60rpm, the 4-nitrophthalic anhydride of 100g, the resorcinol of 118g, the nitrocyclohexane of 50g and the anhydrous cobalt chloride of 25g are mixed, Heat and melt at 120°C, add 110g of zinc chloride to it, react at 140°C for 4.5 hours, then add 1000mL of hydrochloric acid with a concentration of 0.7mol / L, heat and reflux for 30 minutes, filter, and filter the cake with 55°C Washed with water to neutrality, dried at 90° C. for 3 hours to obtain 193.04 g of product (which contained 90.89% of 5-nitrofluorescein and 2.89% of 6-nitrofluorescein);
[0053] (2) Na 2 S and Na 2 SO 3 Dissolve in water to prepare reducing solutions with concentrations of 0.80g / mL and 0.20g / mL respectively, mix the product obtained in step (1) with 1160mL of the reducing solution, react at 70°C for 9 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com