A kind of ce6 derivative, its nano-preparation and its preparation method and application
A nano-formulation and derivative technology, applied in the field of medicine, can solve the problems of low efficiency and insufficient oxygen, and achieve the effect of strong cytotoxicity, good particle size, and improvement of tumor hypoxic microenvironment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 TCE6 molecule synthesis
[0047] Analysis of balance is precisely weighing a certain amount of CE6, dissolved in anhydrous N, N-dimethylformamide (DMF), placed in a 50 ml round bottom flask. A certain amount of NHS, DCC, dissolved in anhydrous DMF, adding a Ce6 solution under stirring, reacted under nitrogen protection, 0 ° C, and at 0 ° C for 24 h. The immunity after the activated Ce6-COOH was removed and placed in a 50 ml round bottom flask, weighing a certain amount of DMAP and TPGS dissolved in anhydrous DMF, adding Ce6-COOH under stirring, protecting, normal temperature conditions in nitrogen The reaction was at 48 h. Among them, the molar ratio of Ce6, TPGS, NHS, DCC, and DMAP is 2: 1: 4: 4: 2. The reaction was completed to obtain a crude product, which was purified by DMF dialysis and water dialysis (dialysis bag.) Was purified, and the final TCE6 solution was frozen to give TCE6, TCE6 pure product was deep green solid, and the yield was 51.92%.
Embodiment 2
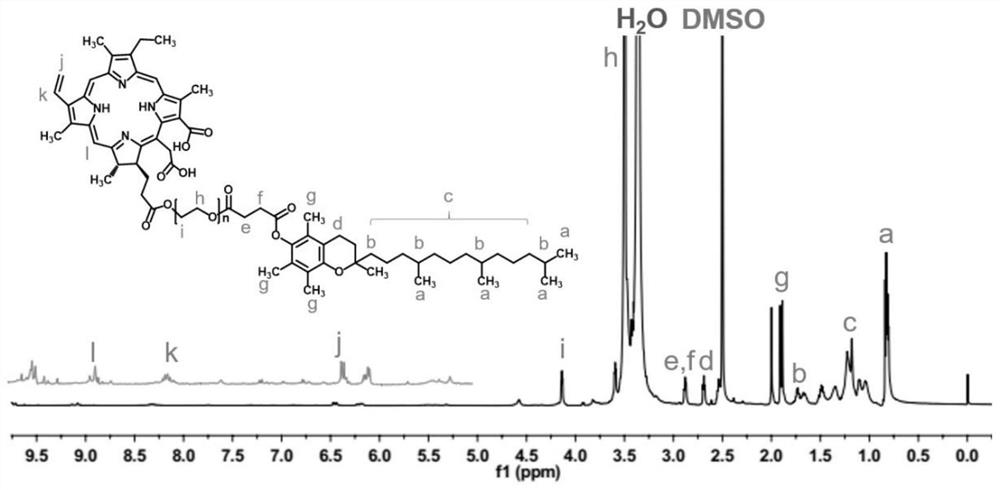
[0048] Example 2 Identification of TCE6 chemical structures by NMR.
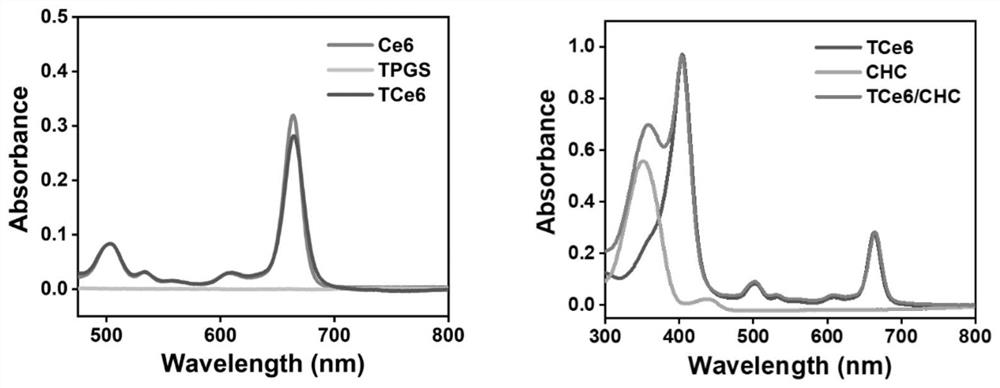
[0049] The TCE6 was given of approximately 5 mg, deuterated dimethyl sulfoxide (DMSO-D6) was dissolved and placed in the nuclear magnetic tube, and the chemical displacement value (PPM) of the recording compound was measured by a 400 MHz nuclear magnetic resonance spectrometer. Such as figure 1 As shown, the nuclear magnetic results may confirm that the peak of the raw material molecules CE6 and TPGS appear in the newly synthetic molecule, confirming the successful synthesis of TCE6; figure 2 On the left, ultraviolet results assisted confirming the successful synthesis of TCE6.
Embodiment 3
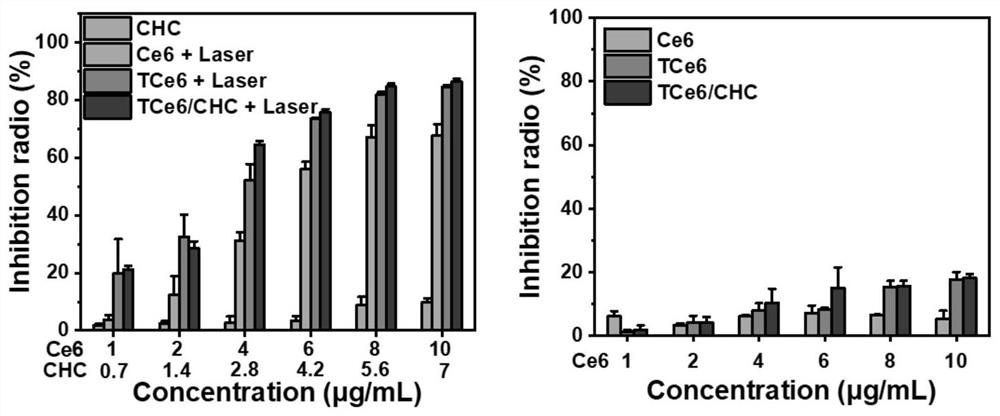
[0050] Example 3 Preparation of TCE6 / CHC NPS.
[0051] Precision weighing TCE6 about 5 mg, dissolved in 50 μl DMF; precisely weighing 15 mg CHC in 150 μL DMF, mixing the TCE6 solution and the CHC solution, stirred for 15 min, the resulting mixed solution droplets were added to the stirred 4ml water, nano aggregate spontaneously The resulting formulation was removed by a dialysis method (dialysis bag, the molecular weight: 1000 Da) was removed, and the impurities were removed by the 0.8 nm filter membrane to obtain the final formulation. UV results figure 2 As shown in the right, the feature of TCE6 and CHCs simultaneously occur in the preparative TCE6 / CHC NPS, and the successful preparation of the formulation is confirmed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com