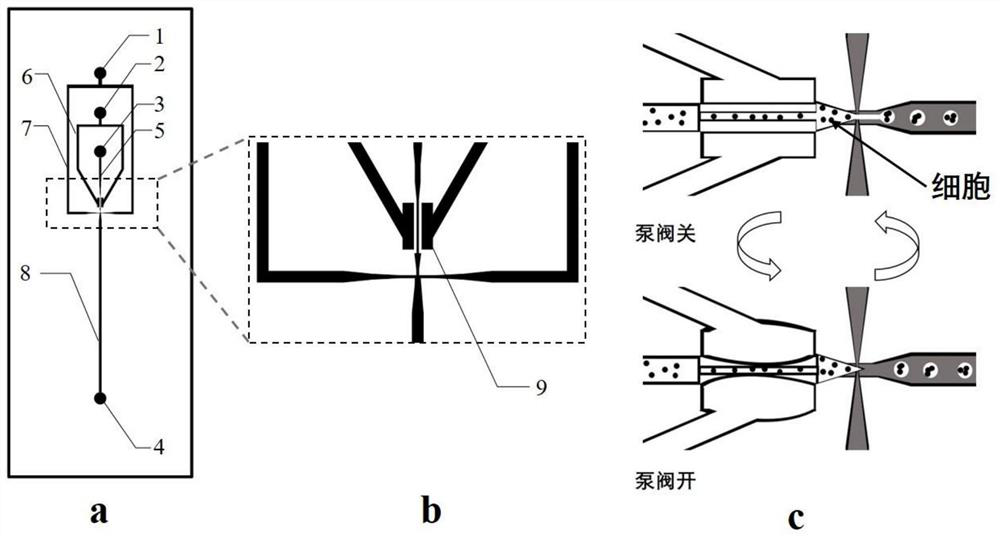
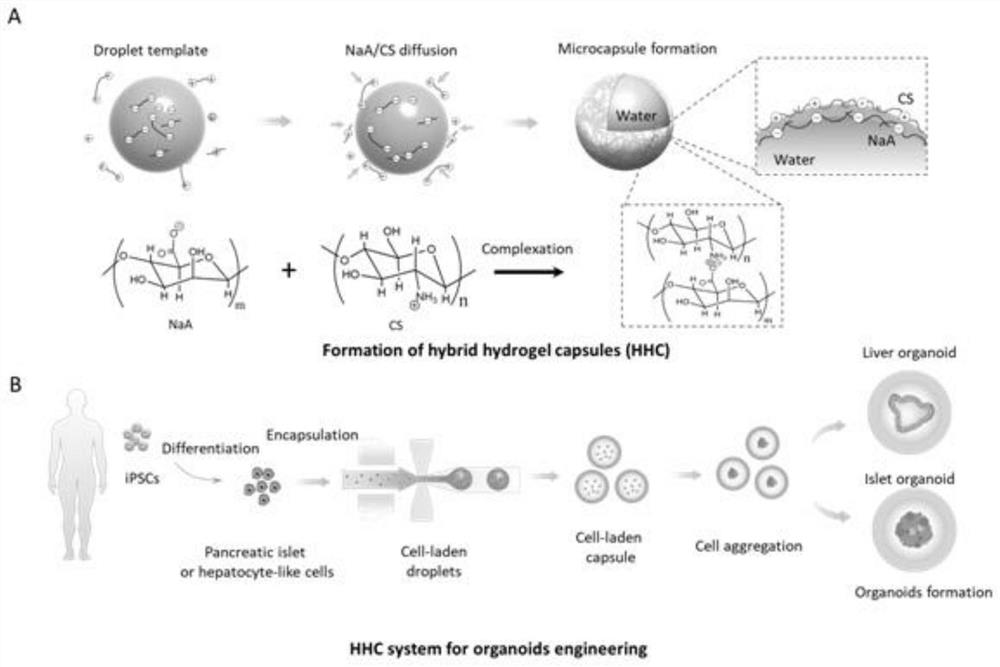
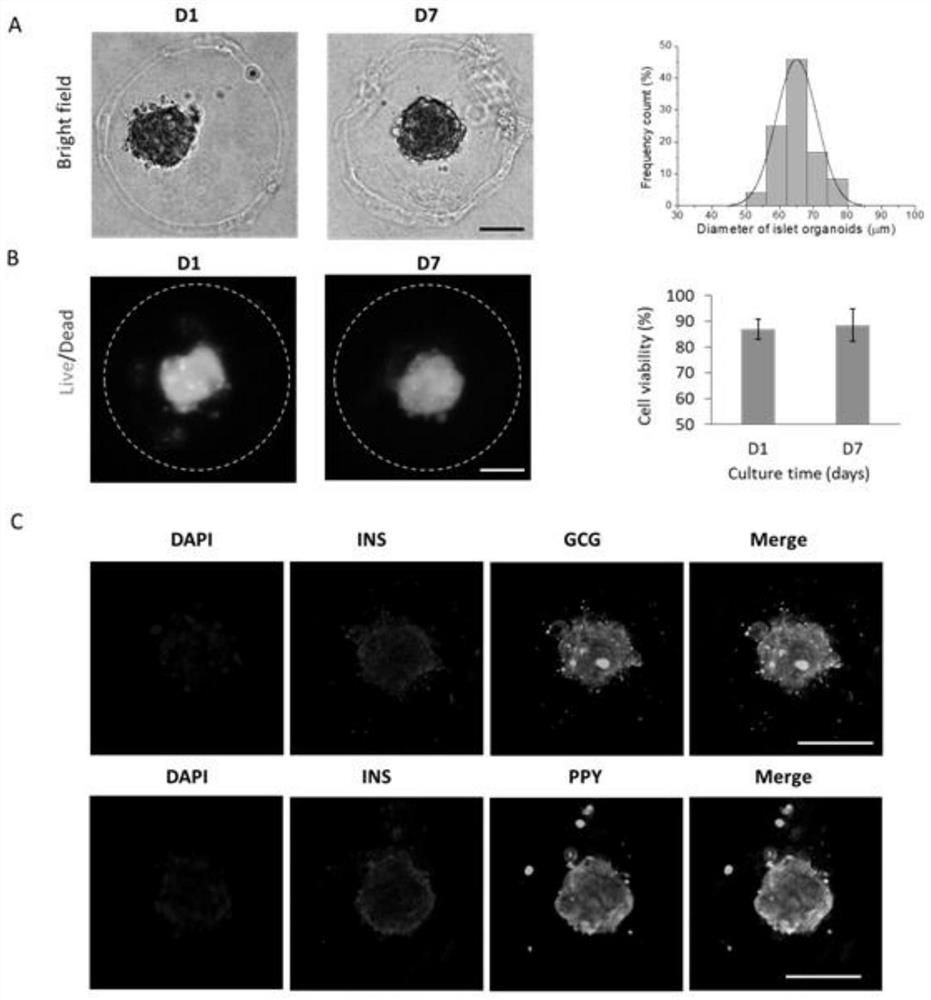
3D organoid engineering method based on two-aqueous-phase droplet microfluidics
An organoid, aqueous two-phase technology, applied in the field of regenerative medicine, to achieve the effect of improving physiological relevance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Aqueous two-phase droplet microfluidic system for islet organoid engineering.
[0045] The specific steps for engineering the islet organoids are:
[0046] (1) Induction of endoderm differentiation: Replace the mTESR1 medium containing human pluripotent stem cells at a density of 50% in the culture plate with DMEM / F12 medium, add 1% of the total volume of B27 supplement, and 1% of the total volume of KSR, GlutaMax accounting for 1% of the total volume and Activin-A factors at a concentration of 80ng / ml were added, and cultured statically for 5 days.
[0047] (2) Induce pancreatic endoderm differentiation: replace DMEM / F12 medium with high-glucose DMEM medium, add 0.5% of the total volume of B27 supplement, the final concentration is 2μM dorsomorphin, 2μM retinoic acid, 10μM SB431542, 5ng / mL basic Fibroblast growth factor (bFGF) and 250nM SANT-1 were cultured statically for 6 days.
[0048] (3) Induction of differentiation of pancreatic endocrine precursor cells: DMEM ...
Embodiment 2
[0053] Aqueous two-phase droplet microfluidic system for islet organoid engineering.
[0054] The specific steps for engineering the islet organoids are:
[0055] (1) Induction of endoderm differentiation: replace the mTESR1 medium containing human pluripotent stem cells at a density of 70% in the culture plate with DMEM / F12 medium, add 1% of the total volume of B27 supplement, and 1% of the total volume of KSR, GlutaMax accounting for 1% of the total volume and Activin-A factors at a concentration of 100 ng / ml were added and cultured statically for 5 days.
[0056] (2) Induce pancreatic endoderm differentiation: replace DMEM / F12 medium with high-glucose DMEM medium, add 0.5% of the total volume of B27 supplement, the final concentration is 2μM dorsomorphin, 2μM retinoic acid, 10μM SB431542, 5ng / mL basic Fibroblast growth factor (bFGF) and 250nM SANT-1 were cultured statically for 6 days.
[0057] (3) Induction of differentiation of pancreatic endocrine precursor cells: DMEM...
Embodiment 3
[0062] Aqueous two-phase droplet microfluidic system for liver organoid engineering.
[0063] The specific steps for engineering the liver organoids are:
[0064] (1) Endoderm induced differentiation: replace the mTESR1 culture medium of human pluripotent stem cells at a density of 50% in the culture plate with 1640+B27 medium, add 80ng / ml of Activin-A factor, and culture for 5 sky.
[0065] The basic component of the 1640+B27 medium is commercialized RPMI-1640 medium, and 1% of the total volume of B27 needs to be added.
[0066] (2) Inducing the differentiation and proliferation of hepatic precursor cells: add HGF and bFGF factors to the 1640+B27 medium and culture for 5 days;
[0067] The final concentration of HGF is 20 ng / ml, and the final concentration of bFGF is 10 ng / ml.
[0068] (3) Production of liver organoids: In order to promote the further differentiation of liver precursor cells, the 1640+B27 medium was replaced with commercial hepatocyte medium HCM, and addit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com