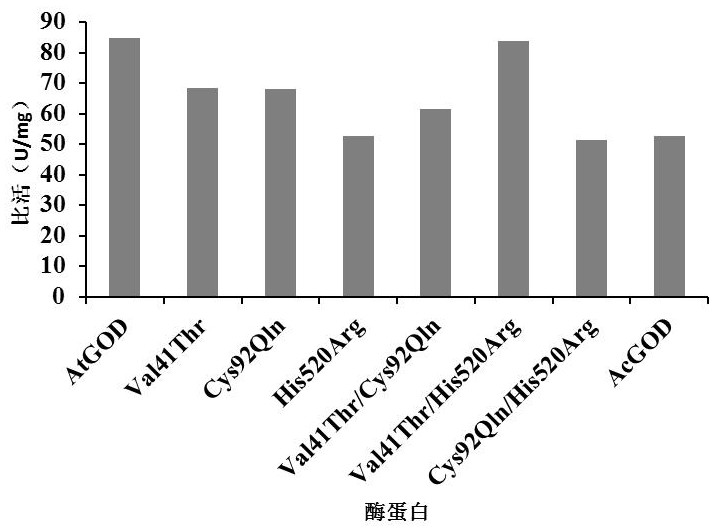
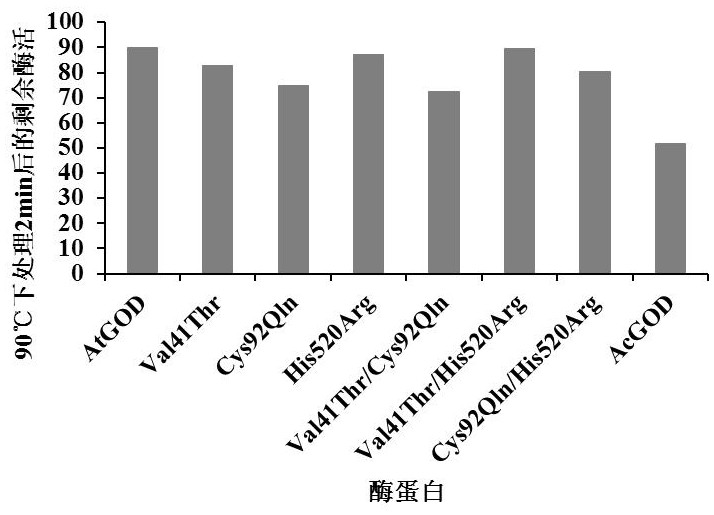
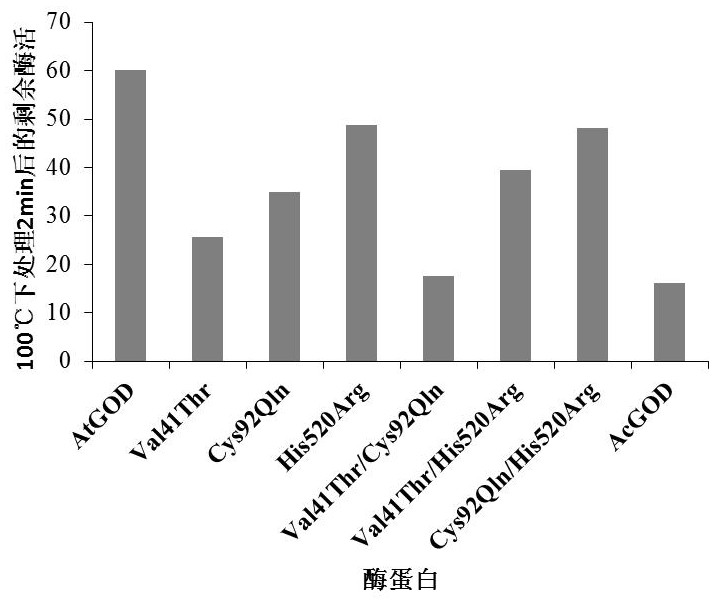
Super-heat-resistant glucose oxidase AtGOD as well as gene and application thereof
A technology of glucose oxidase and sugar oxidase, which is applied in the field of genetic engineering, can solve the problems of rancidity and deterioration of feed, animal harm, and reduce the nutritional value and palatability of feed, so as to reduce the cost of formula, have excellent properties, and meet the physiological characteristics of digestion. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Hyperthermable Glucose Oxidase At Cloning of the GOD-encoding gene
[0038] Aspergillus terreus Aspergillus terreus Genomic DNA and total RNA. Amplification primers were designed and synthesized according to the sequence Eco RI F / not I R:
[0039] Eco RI F: SEQ ID NO: 3, not IR: SEQ ID NO:4.
[0040] PCR amplification was carried out using the above-mentioned genomic DNA and total RNA as templates, respectively. The parameters of the touchdown PCR reaction were: denaturation at 95°C for 5 min; denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec, extension at 72°C for 5 min, 25 cycles, and incubation at 4°C for 10 min. A fragment of about 1800 bp was obtained, which was recovered and connected to the pEazy-T3 vector and sent to BGI Sequencing Company for sequencing.
Embodiment 2
[0041] Example 2 Hyperthermable Glucose Oxidase At Construction of GOD engineering strain
[0042] The expression vector pPIC9 was double digested ( Eco RI+ not 1), simultaneously with the gene encoding superthermostable glucose oxidase At GOD double digestion ( Eco RI+ not I), the digested glucose oxidase gene fragment is connected with the expression vector pPIC9 to obtain a hyperthermogenic glucose oxidase At GOD gene recombinant plasmid pPIC9 -atgod and transform Pichia pastoris GS115 to obtain recombinant Pichia pastoris strain GS115 / atgod .
Embodiment 3
[0043] Example 3 Preparation of Ultra-thermostable Recombinant Glucose Oxidase
[0044] (1) Massive expression of glucose oxidase at shake flask level in Pichia pastoris
[0045] Transformants with good thermal stability and high enzyme activity were screened out, inoculated in 1 L Erlenmeyer flasks with 300 mL of BMGY liquid medium, shaken at 220 rpm at 30°C for 48 h; centrifuged at 4500 rpm for 5 min, discarded Then add 200 mL of BMMY liquid medium containing 0.5% methanol to the cells, induce culture at 30°C, 220 rpm for 48 h. During the induction culture period, methanol solution was added once every 24 hours to compensate for the loss of methanol, so that the methanol concentration was kept at about 0.5%; centrifuged at 12,000×g for 10 minutes, collected the supernatant fermentation liquid, detected the enzyme activity and carried out SDS-PAGE protein electrophoresis analysis .
[0046] (2) Purification of ultrathermostable recombinant glucose oxidase
[0047] Collect ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com