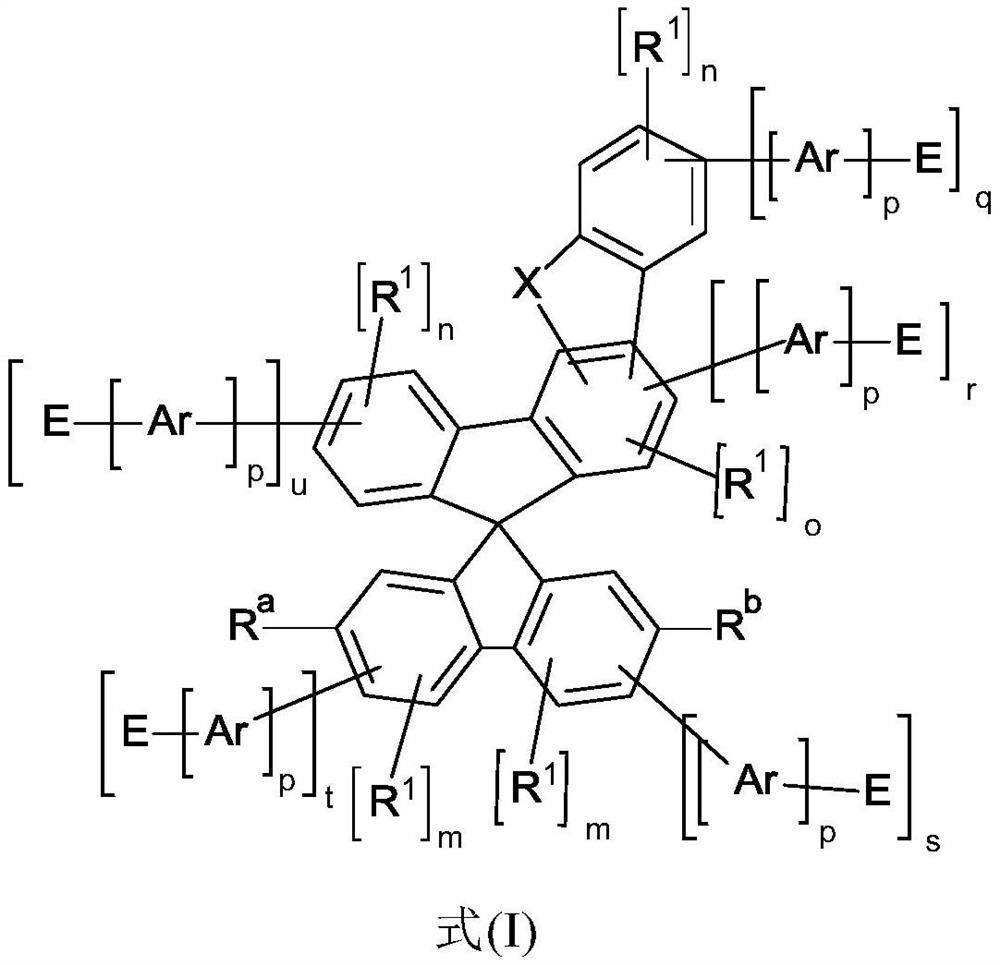
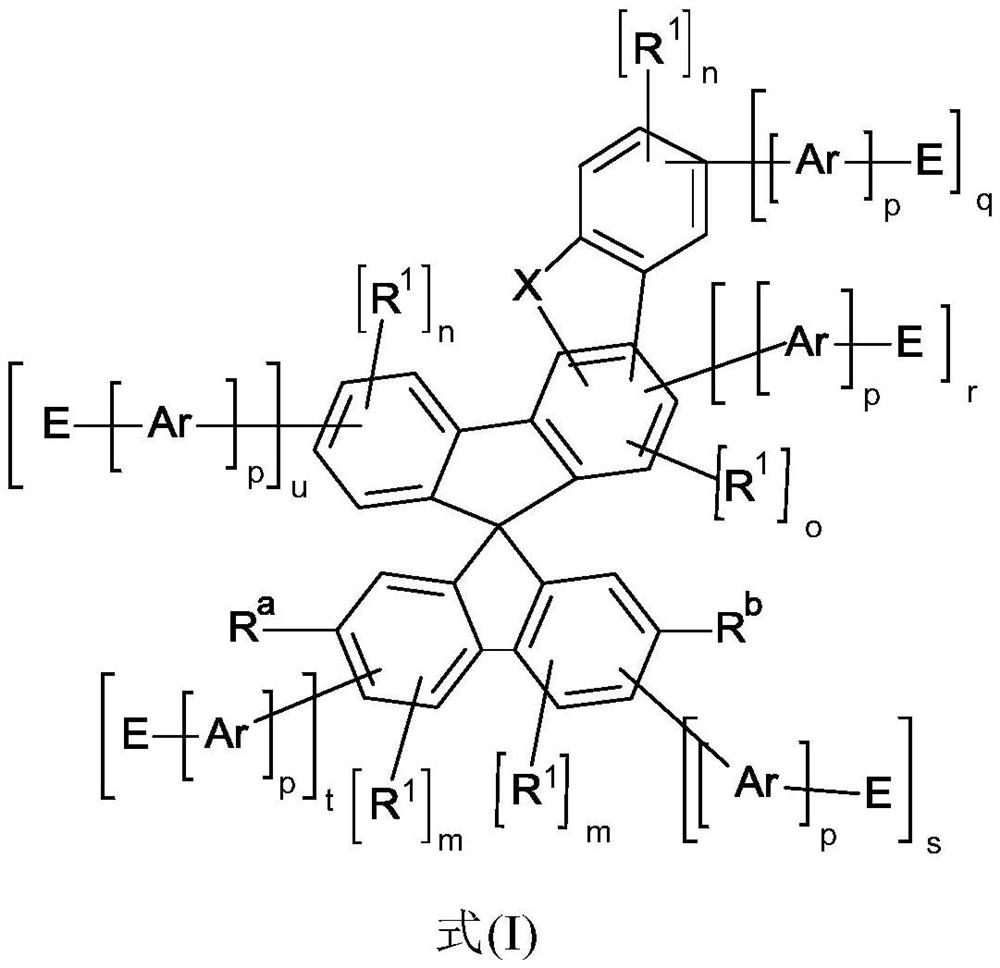
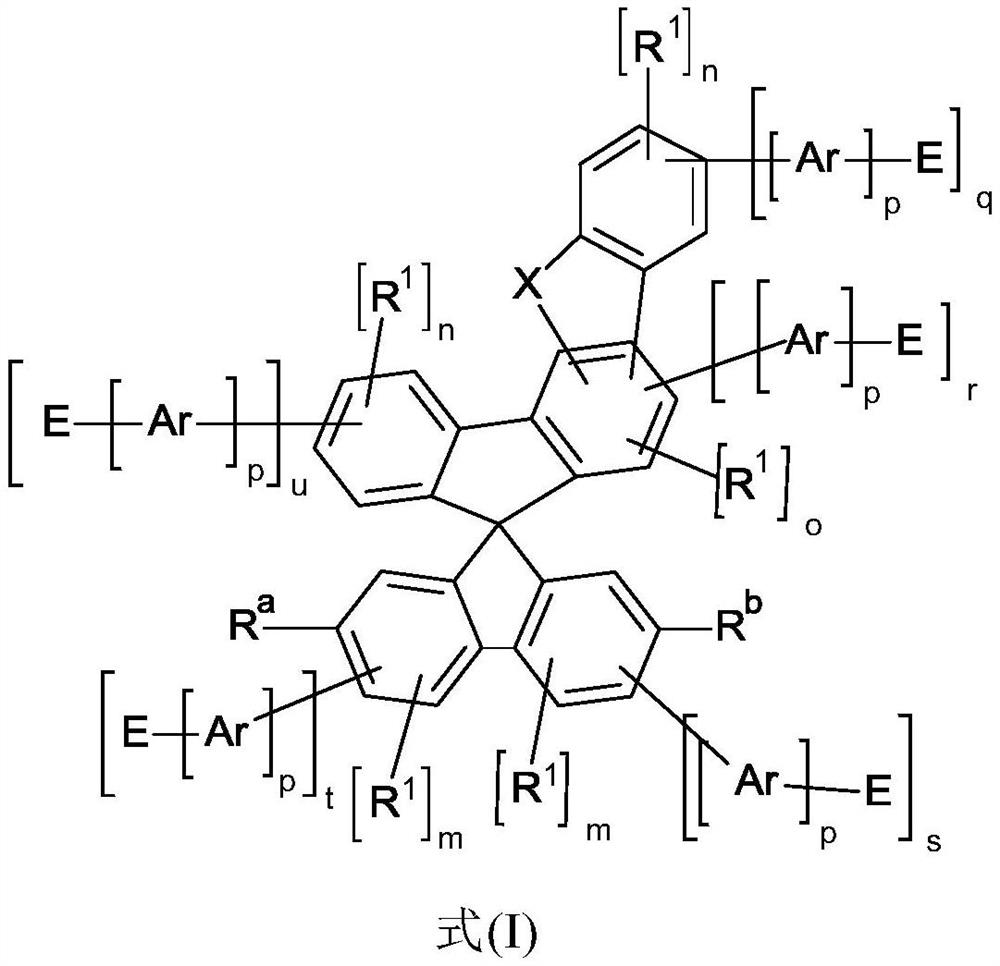
Heterocyclic spiro compounds
A compound and ring system technology, applied in the direction of indium organic compounds, platinum-based organic compounds, platinum-group organic compounds, etc., to achieve good life, high redox stability, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0256]A-1) Example 1: Synthesis of Compounds (1-1) to (1-19)
[0257]
[0258] Synthesis of 4-(2-bromophenyl)dibenzofuran Int-1
[0259] 100 g (462 mmol) of dibenzofuran-4-boronic acid, 106 g (439 mmol) of 1,2-dibromobenzene and 10.7 g (9.2 mmol) of Pd(Ph 3 P) 4 Suspended in 980ml of di in alkane. 979 ml of 2M potassium carbonate solution were slowly added to the suspension, and the reaction mixture was heated at reflux for 16 hours. After cooling, the organic phase is separated off, filtered through silica gel, washed three times with 200 ml of water and then evaporated to dryness. The residue was purified by chromatography on silica gel. Yield: 87 g (270 mmol), 58% of theory, purity >98% by HPLC.
[0260] The following compounds were prepared analogously to the synthesis described for compound Int-1:
[0261]
[0262]
[0263] The following compounds were prepared analogously to the synthesis described for compound Int-29:
[0264]
[0265]
[0266] ...
Embodiment 2
[0281] A-2) Example 2: Synthesis of Compounds (2-1) to (2-4)
[0282] Synthesis of Intermediate Int-29
[0283]
[0284] First, 31 g (90 mmol) of 4-(2-bromophenyl)dibenzofuran were introduced into 300 ml of THF at -78°C. At this temperature, 40 ml of BuLi (2M in hexane) were added dropwise. After 1 hour, 16.9 g (94 mmol) of fluoren-9-one in 200 ml of THF were added dropwise. The batch was allowed to stir overnight at room temperature, added to ice water and extracted with dichloromethane. The combined organic phases were washed with water and dried over sodium sulfate. The solvent was removed in vacuo and the residue was heated without further purification with 94 ml of HCl and 1074 ml of AcOH at reflux at 100° C. overnight. After cooling, the precipitated solid is filtered off with suction, washed once with 100 ml of water, three times with 100 ml of ethanol each and finally recrystallized from heptane. Yield: 23.1 g (57 mmol), 58%; according to 1 H-NMR purity about ...
Embodiment 3
[0301] A-3) Example 3: Synthesis of Compounds 3-1 to 3-5
[0302]
[0303] 110 ml (276 mmol) of n-butyl lithium (2.5 M in hexane) was added dropwise to 137 g (270 mmol) of the bromospiro derivative in 1500 ml of THF cooled to -78 °C. in solution. The reaction mixture was stirred at -78°C for 30 minutes. The mixture was allowed to reach room temperature and cooled to -78°C, then 50 ml (270 mmol) of the mixture of chlorodiphenylphosphine was added rapidly and the mixture was stirred at -70°C for a further 3 hours. After the mixture was warmed to -10°C, it was hydrolyzed with 135 ml of 2N hydrochloric acid. The organic phase is separated off, washed with water, dried over sodium sulfate and evaporated to dryness. Dissolve the residue in 300 ml of CH 2 Cl 2 and stirred with 270 ml of 30% hydrogen peroxide solution at room temperature for 12 hours. The organic phase was separated off and evaporated, the colorless solid was filtered off with suction, washed with n-heptane, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| band gap | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com