16-dehydropregnane dehydropregnenolone acetate compound and preparation method thereof
A technology of diketene alcohol acetic acid and ester compounds, which is applied in the field of organic photochemical synthesis, and can solve problems such as complex operation, environmental pollution, waste of resources and energy consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
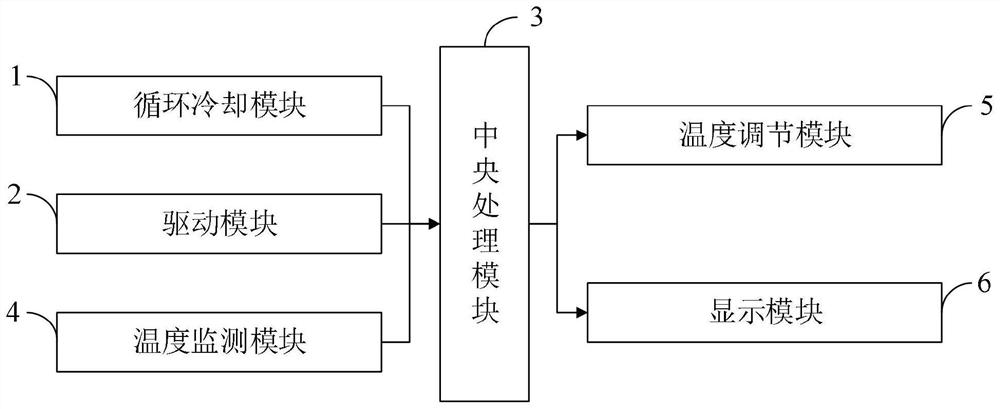
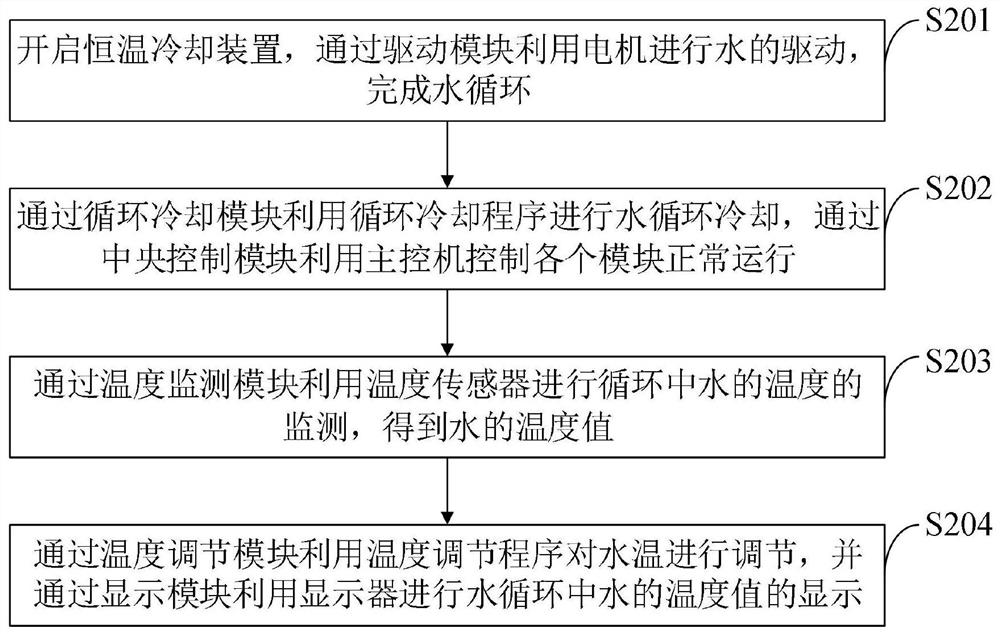
Method used
Image
Examples
Embodiment Construction
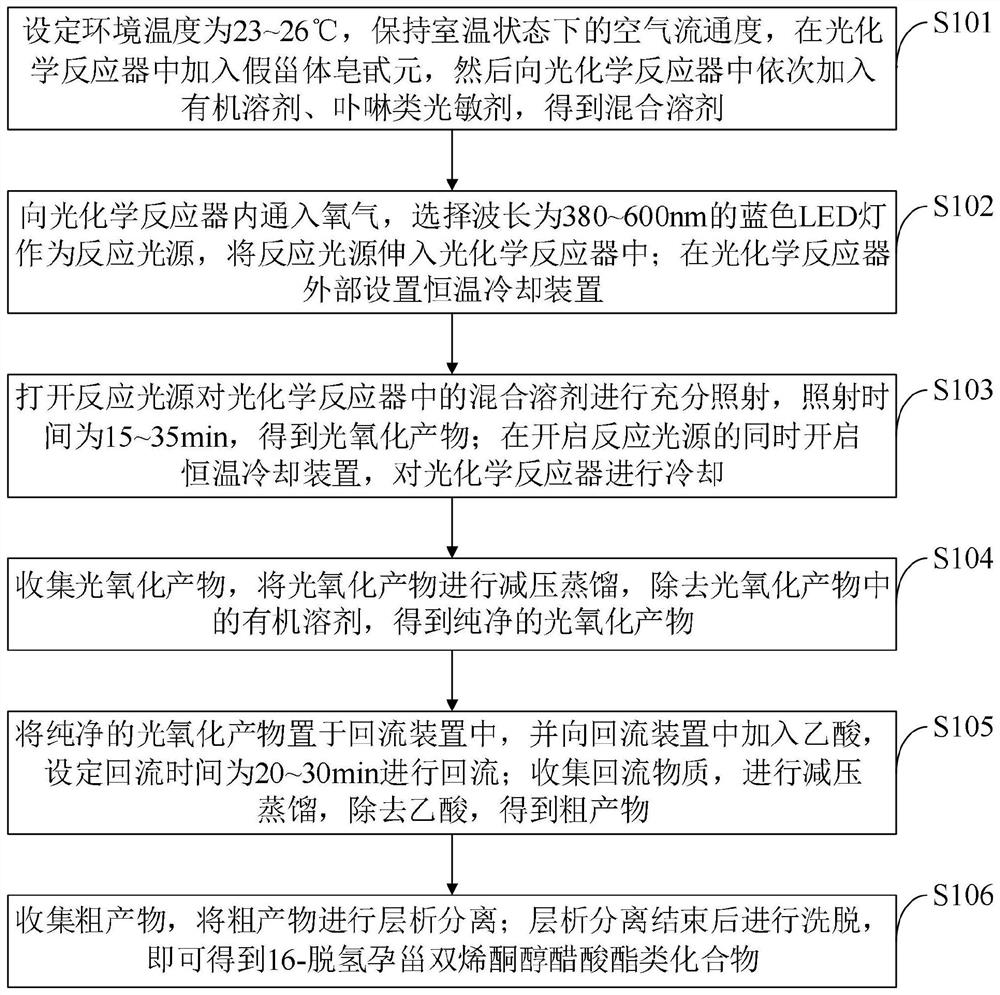
[0042] In order to make the object, technical solution and advantages of the present invention more clear, the present invention will be further described in detail below in conjunction with the examples. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0043] Aiming at the problems existing in the prior art, the present invention provides a 16-dehydropregnone dienone alcohol acetate compound and a preparation method thereof. The present invention will be described in detail below with reference to the accompanying drawings.
[0044] The 16-dehydropregnone dienone alcohol acetate compound provided by the embodiment of the present invention consists of 8-12 parts of pseudosteroidal saponin, 35-50 parts of organic solvent, 3 parts of porphyrin photosensitizer ~5 parts, 10~20 parts of acetic acid.
[0045] The organic solvent provided in the embodiment of the present inventi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com