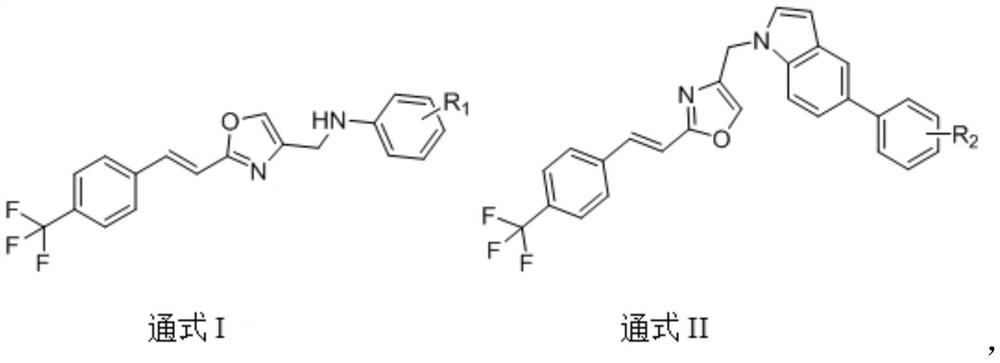
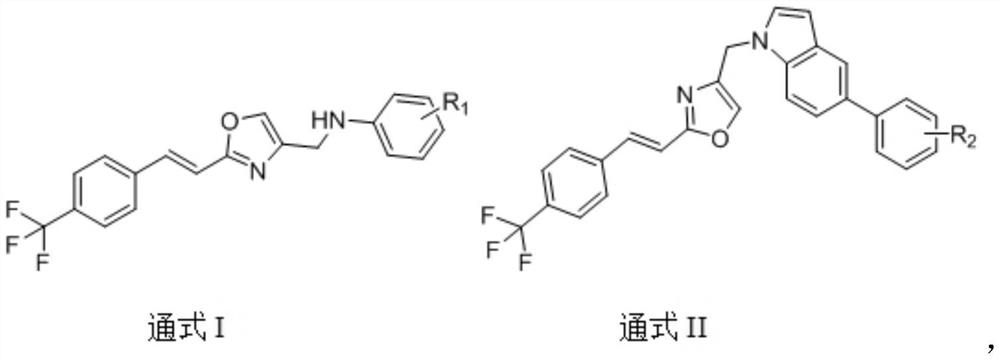
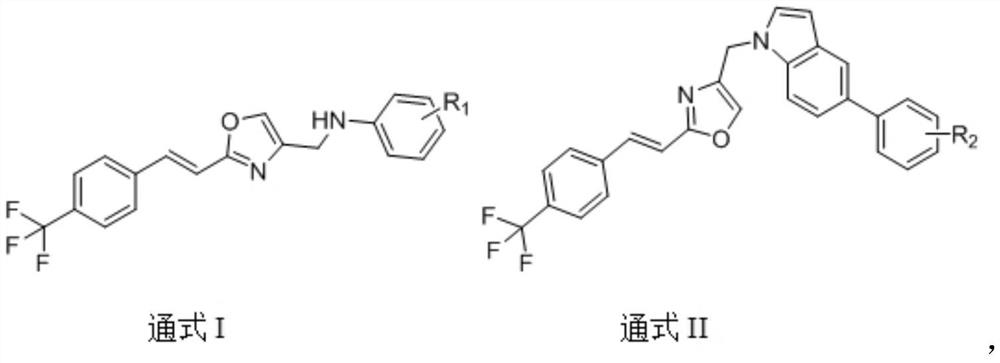
(E)-4-methyl-2-(4-(trifluoromethyl) styryl) oxazole compound as well as preparation method and application thereof
A trifluoromethyl and styrene-based technology, which is applied in the field of medicine, can solve problems that have not been seen, and achieve the effects of good yield, simple and feasible preparation method, and excellent anti-proliferation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Preparation of (E)-N-((2-(4-(trifluoromethyl)styryl)oxazol-4-yl)methyl)aniline (A1).
[0063] a. Preparation of (E)-3-(4-(trifluoromethyl)phenyl)acrylic acid.
[0064] In the 500mL reaction flask, add 1 times the amount (molar amount) 4-(trifluoromethyl) benzaldehyde (30g, 0.17mol), 2 times the amount of pyridine (27.26g, 0.34mol), 1.5 times the amount of malonic acid (26.89 g, 0.25mol), piperidine 10mL, and isopropanol 200mL as solvent, reacted at 90°C, and monitored the reaction progress by thin-layer chromatography. After the reaction was completed, the isopropanol was removed by evaporation under reduced pressure, and water was added to the reaction solution, and the pH value was adjusted to 2. After standing still, the solid was precipitated, and the filter cake was obtained as the product by suction filtration, with a yield of 93.5%.
[0065] b. Preparation of (E)-3-(4-(trifluoromethyl)phenyl)acryloyl chloride.
[0066] Add 1 times the amount of (E)-3-...
Embodiment 2
[0073] Example 2 Preparation of (E)-2-fluoro-N-((2-(4-(trifluoromethyl)styryl)oxazol-4-yl)methyl)aniline (A2).
[0074] The preparation method is the same as (A1).
[0075] White solid, yield: 78.2%. 1 HNMR (600MHz, DMSO-d 6 )δ7.97(s,1H),7.92(d,J=8.0Hz,2H),7.74(d,J=8.0Hz,2H),7.55(d,J=16.5Hz,1H),7.29(d, J=16.4Hz, 1H), 7.02(dd, J=12.1, 8.2Hz, 1H), 6.95(t, J=7.7Hz, 1H), 6.77(t, J=8.5Hz, 1H), 6.56(d, J=5.2Hz, 1H), 5.88(s, 1H), 4.27(d, J=5.9Hz, 2H); 13 CNMR (150MHz, DMSO-d 6 )δ160.11,151.78,149.89,140.43,139.13,136.26,136.14,136.04,133.66,127.81,125.43,124.49,116.46,115.77,114.24,114.09,112.20,38.76;MS(ESI,m / z):363.1122[M+ H] + .
Embodiment 3
[0076] Example 3 Preparation of (E)-3-fluoro-N-((2-(4(trifluoromethyl)styryl)oxazol-4-yl)methyl)aniline (A3).
[0077] The preparation method is the same as (A1).
[0078] White solid, yield: 73.1%. 1 HNMR (600MHz, DMSO-d 6)δ7.97(s,1H),7.89(d,J=6.9Hz,2H),7.71(d,J=8.0Hz,2H),7.54(d,J=16.4Hz,1H),7.31(d, J=10.4Hz, 1H), 7.08(t, J=7.4Hz, 1H), 6.67(d, J=7.7Hz, 2H), 6.55(t, J=7.1Hz, 1H), 6.05(s, 1H) ,4.19(s,2H); 13 CNMR (150MHz, DMSO-d 6 )δ160.31, 160.17, 150.23, 140.15, 139.04, 136.75, 136.27, 133.70, 130.02, 127.79, 125.41, 116.39, 108.42, 101.95, 101.78, 98.46, 136.26, 45.93 m / z H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com