Synthesis method of diphenyl pyrazine derivative
A diphenylpyrazine and synthesis method technology, applied in the direction of organic chemistry, etc., can solve problems such as difficult long-term storage, potential safety hazards, instability of diphenylpyrazine, etc., and achieve high feasibility, good safety, and reasonable design Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
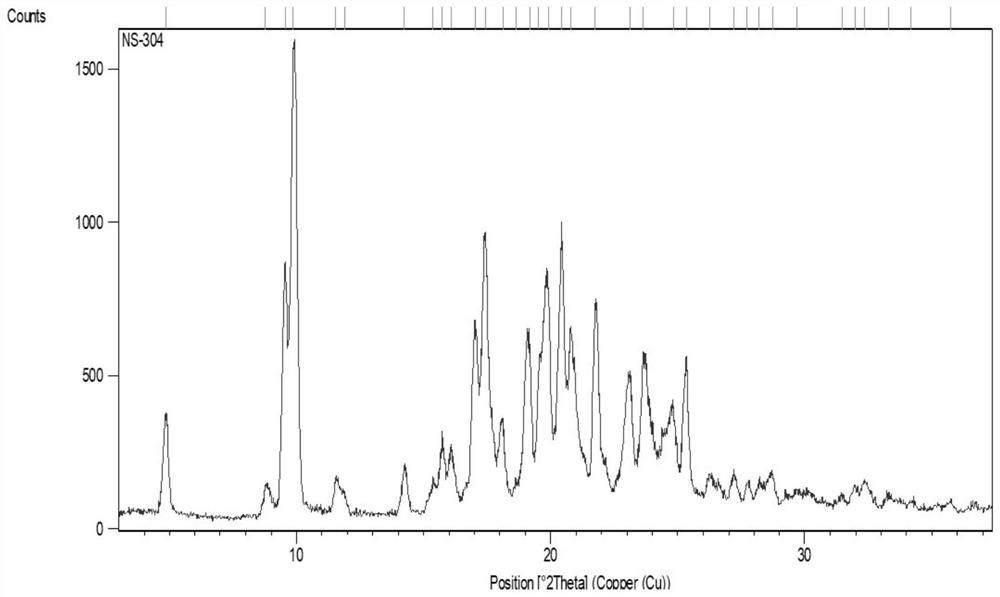
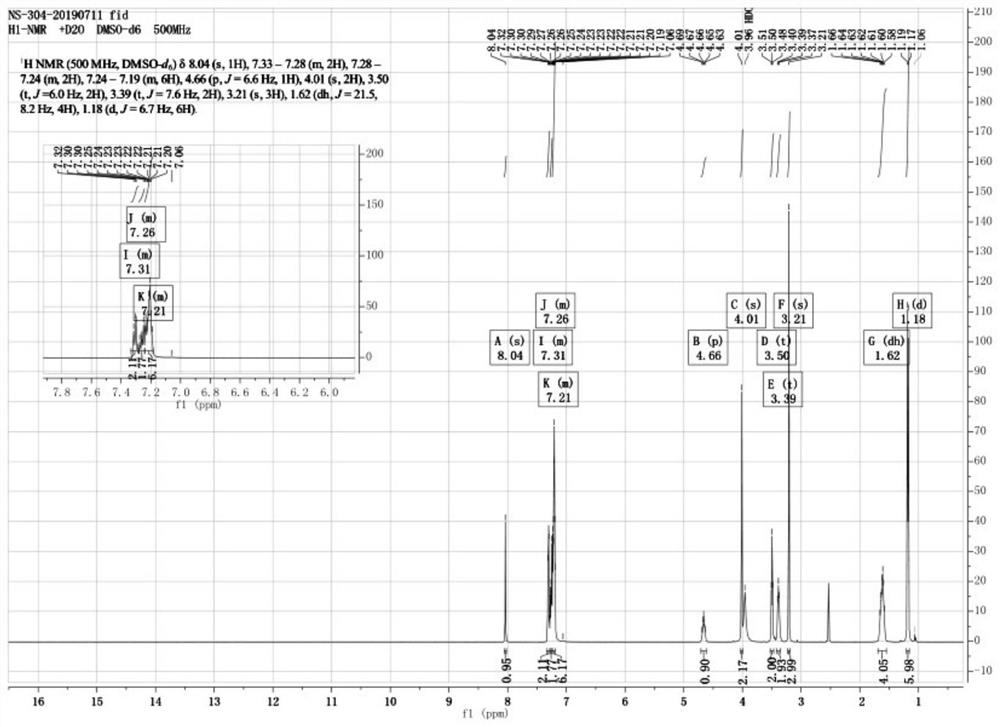
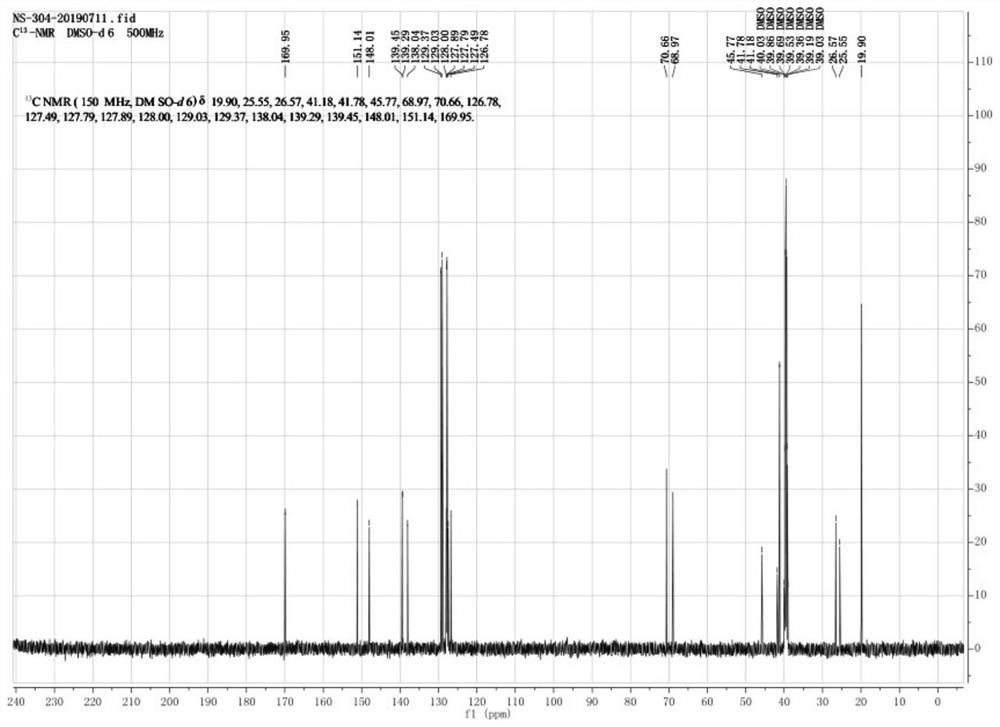
Image
Examples
Embodiment 1
[0039] Synthesis of 5,6-diphenyl-2-hydroxypyrazine (compound NS-300)
[0040]
[0041] Reaction steps:
[0042] Add methanol (350mL) to a 1000mL three-necked flask equipped with a condenser, add benzil (35.02g) and glycinamide hydrochloride (19.75g, 1.1eq), cool in an ice-water bath to 10°C, and slowly add 12N Sodium hydroxide solution (30mL), temperature control ≤ 30°C, after dropwise addition, turn to oil bath for heating, heat up to 70°C for reflux reaction for 12h, HPLC detects that raw materials disappear, stop heating, turn on cooling and cool down to 10°C, add ice to the system Acetic acid was used to adjust the pH to 6-7, and the retested pH remained unchanged. A large amount of light yellow solid precipitated out, and the bright yellow solid was obtained by suction filtration, which was air-dried at 70°C and directly put into the next step without purification.
Embodiment 2
[0044] Synthesis of 5-bromo-2,3-diphenylpyrazine (compound NS-301)
[0045]
[0046] Reaction steps:
[0047]Add acetonitrile (350mL) to a 500mL three-necked flask equipped with a condenser, stir and add NS-300 (30.00g), add phosphorus oxybromide (103.92g, 3.0eq), stir and heat up to 70°C, react for 30h, and detect by HPLC When the reaction was complete, the reaction solution was slowly added to 850mL of water to quench the reaction, and a large amount of light purple solid was precipitated, which was filtered with suction, and the filter cake was beaten with dilute NaOH solution, adjusted to neutrality, and 33.46g of solid was obtained by suction filtration. Yield about 89%. ESI-MS:m / z=313.0(M+H) + . 1 H NMR (500MHz, DMSO-d 6 )δ8.84 (s, 1H), 7.43-7.30 (m, 10H).
Embodiment 3
[0049] Synthesis of N-isopropyl-5,6-diphenylpyrazin-2-amine (Compound NS-302)
[0050]
[0051] Reaction steps:
[0052] Add dioxane (250mL), NS-301 (30.03g), isopropylamine (8.62g, 1.5eq), KI (4.80g, 0.3eq) and potassium carbonate (41.14g, 2.0eq ), stirred evenly, heated to 40-45° C. for 12 hours, and TLC monitored that the reaction was complete. The reaction mixture was cooled to around 10°C, quenched with water (100 mL) and extracted with dichloromethane. The combined organic layers were washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was distilled off to obtain 20.41 g of a solid. The yield is about 73%. ESI-MS:m / z=290.4(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com