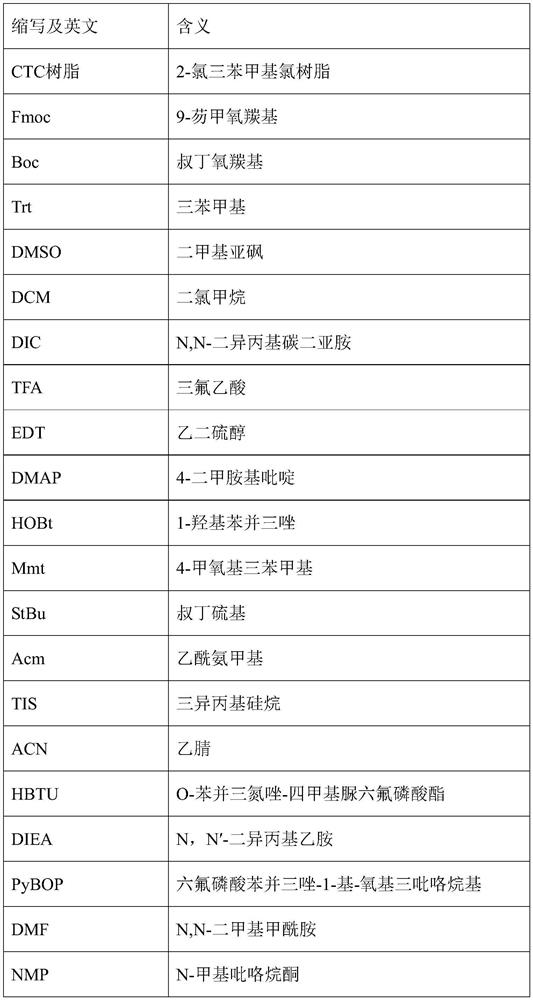
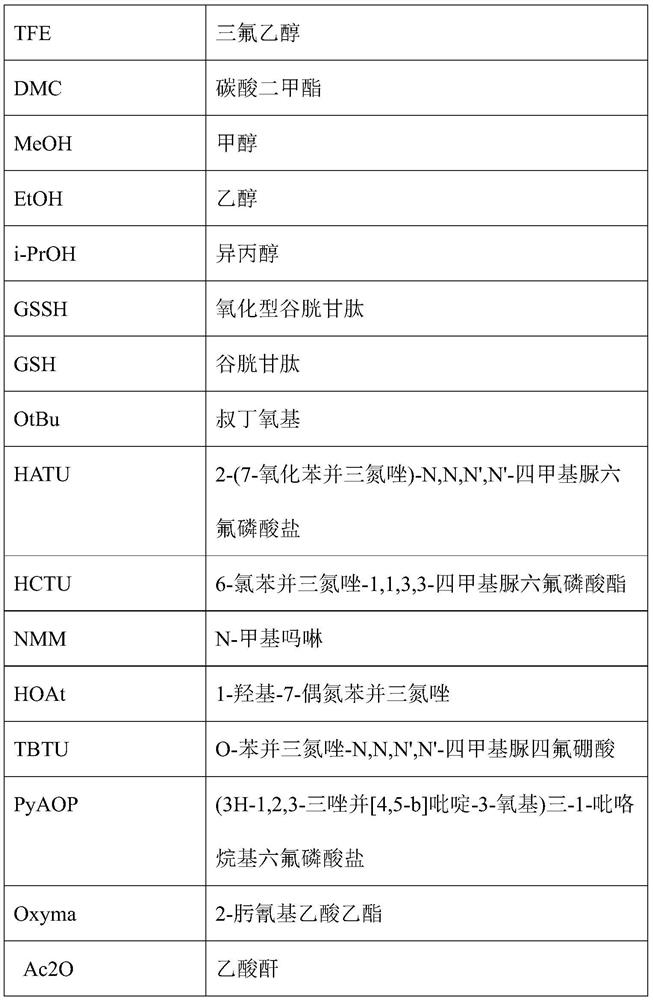
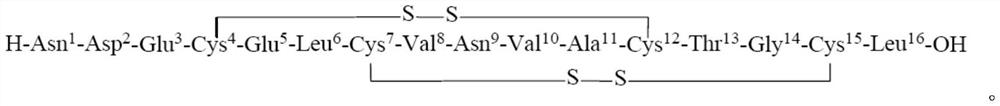Preparation method of plecanatide
A technology of plecanatide and peptide fragments, which is applied in the field of preparation of polypeptide compound plecanatide, can solve the problems of poor direct coupling effect of N-terminal amino acids, long reaction time, cumbersome processing, etc., and achieves strong practical application value, The effect of low separation and purification difficulty and simple solution components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: A kind of preparation method of plecanatide
[0052] 1. Preparation of BocAA1-4OH peptide fragment
[0053] (1) BocAA1-4OH peptide fragment sequence
[0054] Boc-Asn(trt)-Asp(OtBu)-Glu(OtBu)-Cys(Acm)-OH
[0055] (2) Synthesis of Fmoc-Cys(Trt)-CTC resin
[0056] Weigh 2.76g of 2-chlorotrityl chloride resin (CTC resin) with a degree of substitution of 1.1mmol / g into the polypeptide reactor, and add 27ml of DCM at the same time to wash and swell the resin for 1 hour. Weigh Fmoc-Cys(Acm)-OH (9mmol, 3.80g), add 19ml DCM to dissolve, add the dissolved reaction solution to the resin, and after the resin and the reaction solution are stirred evenly, add DIEA (13.5 mmol, 3.38ml), reacted at 25°C for 2 hours. After the reaction was completed, 2.21 ml of methanol was further added to the reaction solution to block unreacted active sites, and the reaction was performed for 1 hour. After the reaction was completed, the solution was drained, and the resin was washe...
Embodiment 2
[0080] Embodiment 2: A kind of preparation method of plecanatide
[0081] 1. Preparation of BocAA1-6OH peptide fragment
[0082] (1) BocAA1-6OH peptide fragment sequence
[0083] Boc-Asn(trt)-Asp(OtBu)-Glu(OtBu)-Cys(Trt)-Glu(OtBu)-Leu-OH
[0084] (2) Synthesis of Fmoc-Leu-CTC resin
[0085] Weigh 2.73g of CTC resin with a degree of substitution of 1.1mmol / g and add it into the polypeptide reactor, while adding 27ml of DCM to wash and swell the resin for 1 hour. Weigh Fmoc-Leu-OH (9mmol, 3.18g), add 19ml DCM to dissolve, add the dissolved reaction solution to the resin, after the resin and the reaction solution are stirred evenly, add DIEA (13.5mmol, 3.38g) to the resin reaction solution ml), react at 25°C for 2 hours. After the reaction was completed, 2.3 ml of methanol was further added to the reaction solution to block unreacted active sites, and the reaction was performed for 1 hour. After the reaction was completed, the solution was drained, and the resin was washed 4...
Embodiment 3
[0109] Embodiment 3: A kind of preparation method of plecanatide
[0110] 1. Preparation of BocAA1-8OH peptide fragment
[0111] (1) BocAA1-8OH peptide fragment sequence
[0112] Boc-Asn(trt)-Asp(OtBu)-Glu(OtBu)-Cys(Trt)-Glu(OtBu)-Leu-Cys(Acm)-Val-OH
[0113] (2) Synthesis of Fmoc-Val-CTC resin
[0114] Weigh 2.75g of CTC resin with a substitution degree of 1.1mmol / g and add it into the polypeptide reactor, while adding 27ml of DCM to wash and swell the resin for 1 hour. Weigh Fmoc-Val-OH (9mmol, 3.08g), add 19ml DCM to dissolve, add the dissolved reaction solution to the resin, and after the resin and the reaction solution are evenly stirred, add DIEA (13.5mmol, 3.36 ml), react at 25°C for 2 hours. After the reaction was completed, 2.3 ml of methanol was further added to the reaction solution to block unreacted active sites, and the reaction was performed for 1 hour. After the reaction was completed, the solution was drained, washed 4 times with 27 ml, and the resin was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com