Stable candesartan cilexetil pharmaceutical solid composition and preparation method thereof
A technology of candesartan cilexetil and solid composition, which is applied in the field of candesartan cilexetil pharmaceutical solid composition and its preparation, can solve the problems of difficult pH regulator full contact, uncertainty of inhibitory effect, and difficulty in practical application and other problems, to achieve the effects of not easy crystal transformation, lower energy consumption, and strong productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
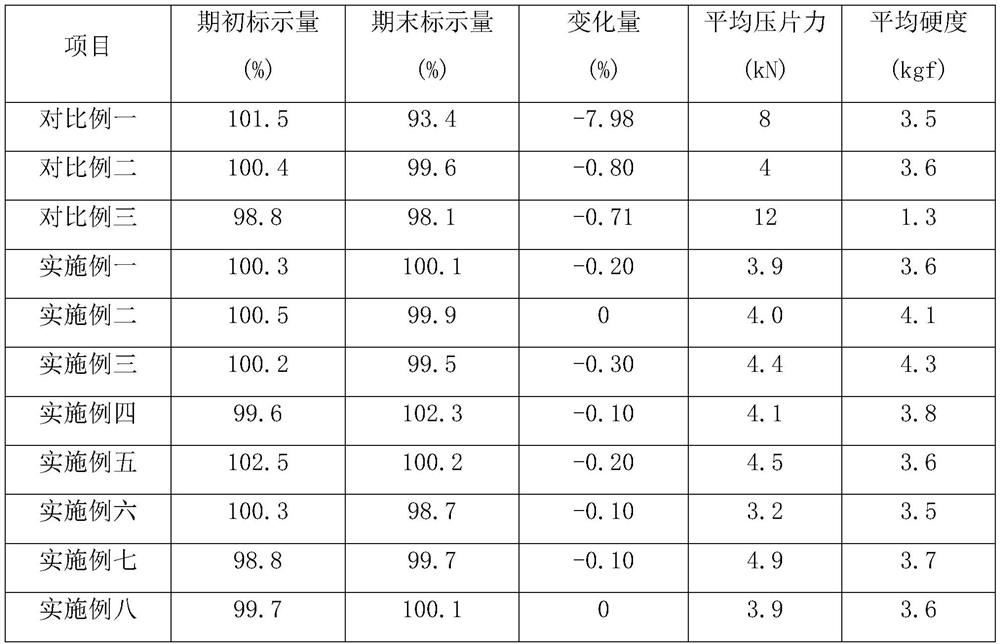
Examples
Embodiment 1
[0051] 2 parts of Cantaisa, 0.2 glycerol, 0.2 sodium citrate, 6000 polyethylene glycol, 93.6 lactose, 20 corn starch, 4 hydroxypropylene, 5.6 copies of carboxymethylcarboxymethyl. 0.4 sq.
[0052] The preparation steps of the stable ceiling salty ester drug solid composition of the first example are as follows:
[0053] Step 1: 2 purified water formation in the mixture of sodium sodium citrate, glycerol and ceilica.
[0054] Step 2: The paspect is added to a solution made of hydroxypropylene cellulose, polyethylene glycol, and stirred at room temperature;
[0055] Step 3: Use a atomized spray gun into the fluidized bed containing lactose, corn starch and produce particles at a material temperature of 20 to 50 ° C;
[0056] Step 4: Take a tablet drug under the pressure of carboxymethylcalfolium calcium and magnesium stearate after drying particles.
Embodiment 2
[0058] 2 parts of Canthaisa, 0.4 glycerol, 0.4 sodium citrate, 6000 polyethylene glycol, 89.2 lactose, 20 corn starch, 4 hydroxypropylene, 5.6 copies of carboxymethyl cellulose calcium 0.4 sq.
[0059] Step 1: 4 purified water formation of a paste in a mixture of sodium sodium citrate, glycerol and ceilifier, and other steps of preparing a stable celycetha drug solid composition in the second embodiment. Similarly to the first example, details are not described herein.
Embodiment 3
[0061] 4 parts of Cantaisa, 0.4 glycerol, 0.4 sodium citrate, 6000 polyethylene glycol, 91.2 lactose, 20 parts of corn starch, 4 hydroxypropylene, 5.6 copies of carboxymethylca calcium 0.4 sq.
[0062] Step 1: 4 purified water formation of a psychedelic substrate to the mixture of sodium sodium citrate, glycerol and celycetan, and other steps of preparing a stable celycetha drug solid composition of the third embodiment Similarly to the first example, details are not described herein.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com