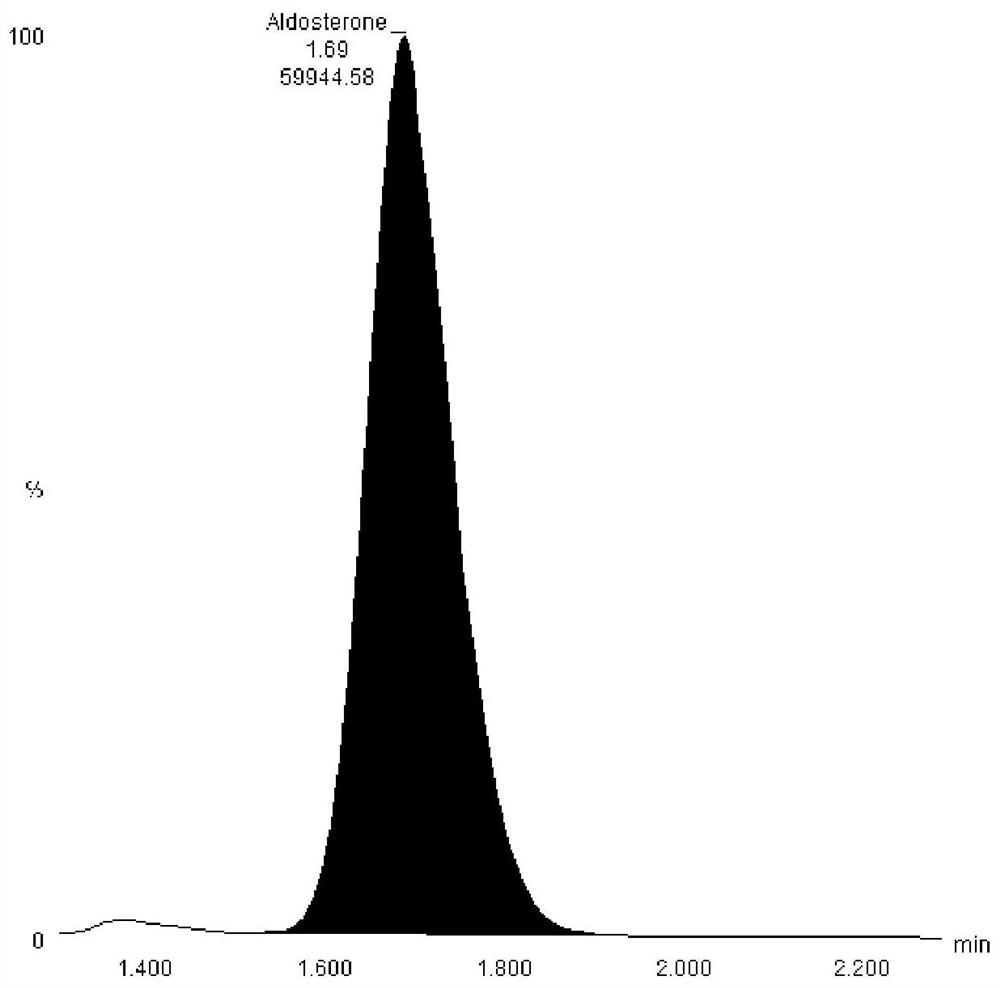
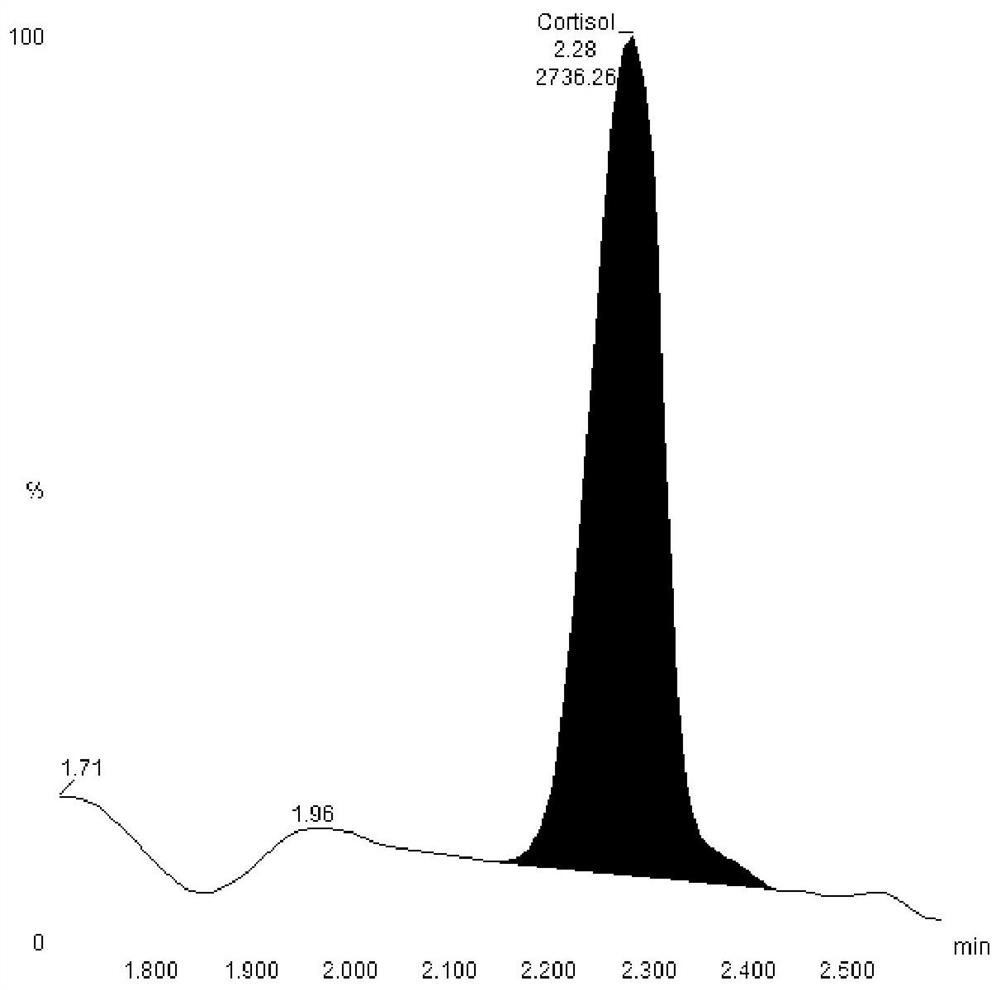
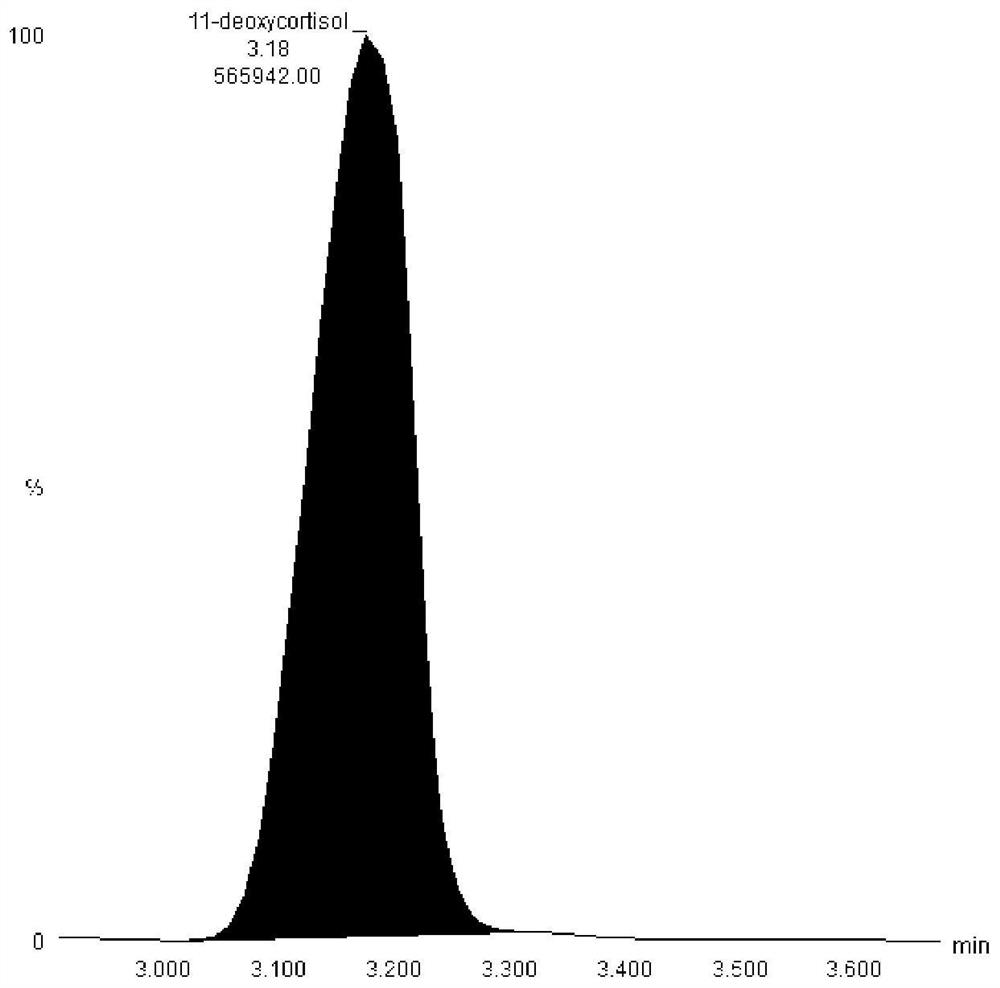
Hormone mass spectrometric detection method based on antibody coupling magnetic bead enrichment technology
A technology of mass spectrometry detection and antibody coupling, which is applied in mass spectrometry analysis, measuring devices, instruments, etc., can solve the problems of narrow linear range, limited application of liquid mass spectrometry and poor specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: The antibody contains small molecular compounds of endogenous hormones.
[0029] Reagent preparation:
[0030] Four antibodies of cortisol, testosterone, progesterone and aldosterone were selected, and the antibodies were purchased from Thermo; methanol was purchased from Merck, and methanol was mixed with water to prepare an 80% aqueous methanol solution;
[0031] Experimental equipment preparation:
[0032] 1.5ml centrifuge tube self-made magnetic stand; 1.5ml centrifuge tube; Vortex shaker (Scientific Industries * Vortex-Genie2 company, Vortex-Genie 2); Waters TQS mass spectrometer (Waters company);
[0033] experiment procedure:
[0034] Take 5μl antibody (aldosterone, progesterone, testosterone or cortisol) into 1.5ml centrifuge tube, add 100μl 80% methanol aqueous solution, vortex in vortex shaker for 3min, centrifuge at 12000g 4℃ for 10min, take supernatant Put 50 μl into a 96-well plate, then add 50 μl of water, mix well and detect on Waters TQS. ...
Embodiment 2
[0051] Example 2: Both 0.1M glycine pH2.8 eluent and 20% methanol solution cannot effectively remove endogenous hormones on antibody-coupled magnetic beads
[0052] Reagent preparation:
[0053] Glycine was purchased from Sigma, prepared as 0.1M glycine pH2.8; phosphate buffer saline (PBS) was purchased from Sigma, deionized water was purchased from Merck; From sigma, add water to prepare 50mM Tris-HCl, pH7.4, and carboxyl magnetic beads were purchased from Yingruicheng Biochemical Technology Co., Ltd. Other reagents are with embodiment one.
[0054] Experimental instrument preparation: same as implementation case 1
[0055] experiment procedure:
[0056] 1. Antibody magnetic bead coupling: mix the magnetic beads evenly, take 10mg magnetic beads and add them to a 1.5mL EP tube, remove the supernatant by magnetic separation on the magnetic stand, add deionized water and mix evenly, remove the supernatant by magnetic separation , and repeat 2 more times. Add 100 μL PBS to...
Embodiment 3
[0066] Example 3: 80% methanol can effectively remove endogenous hormones on antibody-coupled magnetic beads without affecting antibody activity. 10% methanol is used as an enrichment system and mixed with 4 kinds of antibodies to achieve better enrichment effect
[0067] Reagent preparation:
[0068] All hormone compounds and internal standards were purchased from Sigma, and other reagents were the same as those in Example 1 or 2, and 10% methanol was used to prepare internal standards (Table 4) and calibrator (Table 5) respectively.
[0069] Table 4 internal standard concentration of each compound
[0070]
[0071] Table 5 Concentration of each compound in calibrator
[0072]
[0073]
[0074] Other reagents and experimental equipment are prepared with embodiment one or two.
[0075] experiment procedure:
[0076] The method of preparing antibody-coupled magnetic beads is the same as that described in Example 2.
[0077] 1. Removal of endogenous hormones on anti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com