Preparation method of lenvatinib and preparation method of lenvatinib intermediate
A solvent and compound technology, which is applied in the field of preparation of lenvatinib and intermediates, can solve the problems of single preparation method of lenvatinib, and achieve the effects of low toxicity, high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104]
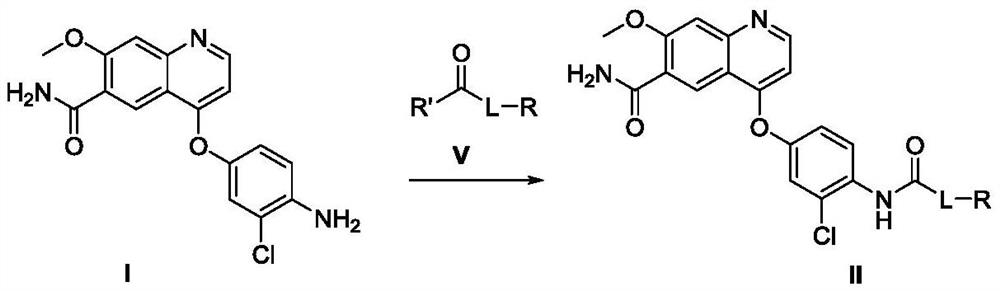
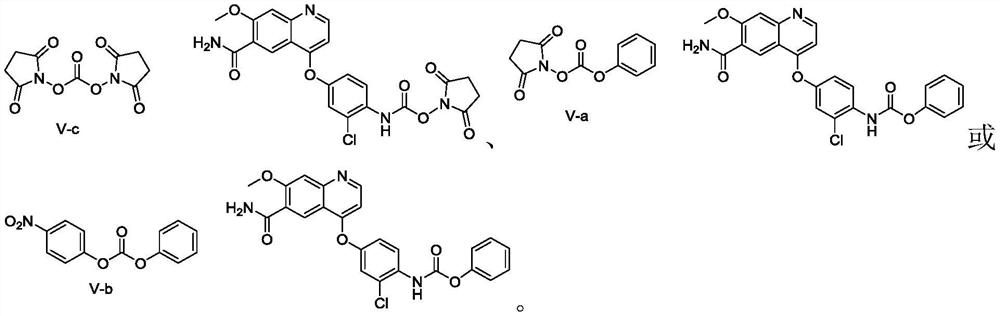
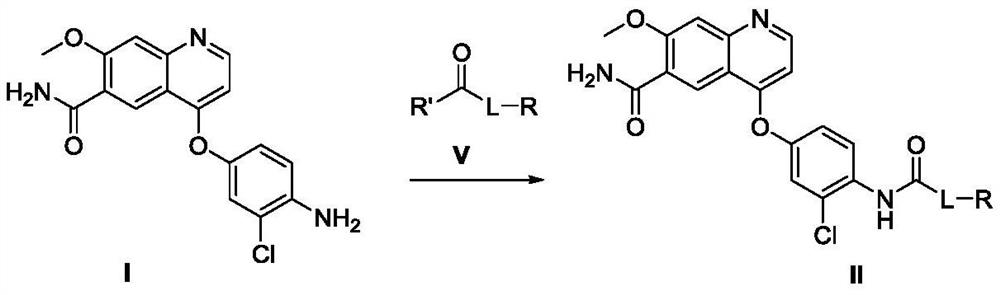
[0105] In a 250mL three-necked flask equipped with a reflux condenser, add N,N'-bis-succinimide carbonate (also called: succinimide carbonate, hereinafter replaced by succinimide carbonate) (6.85 g, 26.76mmol) and add 50mL DMF to dissolve at room temperature, and replace the gas in the reaction bottle with a nitrogen balloon for 3 times, keep the nitrogen atmosphere in the bottle, and heat to an inner temperature of 40 degrees. After stirring and dissolving, 4-(4-amino-3-chlorophenyloxy)-7-methoxy-quinoline-6-carboxamide (5.00 g, 14.55 mmol) was added in batches and stirred for 1 hour. The reaction is complete (detecting 4-(4-amino-3-chlorophenyloxy)-7-methoxy-quinoline-6-carboxamide with HPLC is less than 0.2% (area normalization method). The step formed The intermediate was unstable, and during separation and purification, it was partially degraded to the starting material), cooled to room temperature, added cyclopropylamine (2.29 g, 51 mmol, 3.5 equivalents), an...
Embodiment 2
[0111] Adopt reaction conditions and method as embodiment 1, difference only is to use different solvents to replace DMF; The result is as follows:
[0112] serial number Solvent type Crude reaction yield Crude purity 1 DMF 103.2% 99.2% 2-1 DMSO 90% 95.5% 2-2 THF 70% 45.2% (incomplete conversion of raw materials)
[0113] Adopt reaction condition and method as embodiment 1, difference only is to use different temperature to react
[0114] serial number temperature reflex Crude reaction yield Crude purity 1 40℃ 103.2% 99.2% 2-3 55℃ 99% 81.2% (reaction becomes impurity) 2-4 35℃ 42% 32.5% (incomplete conversion of raw materials)
[0115] Adopt the reaction condition and method as embodiment 1, difference only is that the equivalent number of succinimidyl carbonate is different
[0116] serial number Succinimide carbonate equivalent Crude reaction yield Crude purity 1 1.84eq...
Embodiment 3
[0118] The synthetic method of succinimide-phenol carbonate:
[0119]
[0120]N-hydroxysuccinimide (11.5g, 100mmol) and diethylaniline (16.4g, 110mmol) were dissolved in dichloromethane (115mL) at room temperature, and the temperature was lowered to -20°C, and chloroformic acid benzene was added dropwise Ester (17.2 g, 110 mmol) was added dropwise over 30 minutes. After the dropwise addition was completed, the temperature was gradually raised to room temperature, and stirred at room temperature for 1 hour. Add 50 mL of dilute hydrochloric acid (1.0 M) to wash once, brine (50 mL) once, and dry over anhydrous sodium sulfate. Concentration under reduced pressure gave a white solid. The white solid was dissolved with dichloromethane (100 mL) under reflux, and n-heptane was added dropwise to precipitate white crystals, which were stirred at room temperature for 3 hours to grow crystals. The crystals were filtered out and dried in vacuo to obtain pure 4-nitrobenzene-phenol car...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com