Alkaloid streptindole derivative, preparation thereof, and application of alkaloid streptindole derivative in prevention and control of plant viruses and pathogenic bacteria
A technology for plant pathogens and derivatives, which is applied in the fields of chemicals for biological control, botanical equipment and methods, applications, etc., and can solve the problems of few therapeutic agents and few plant virus inhibitors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
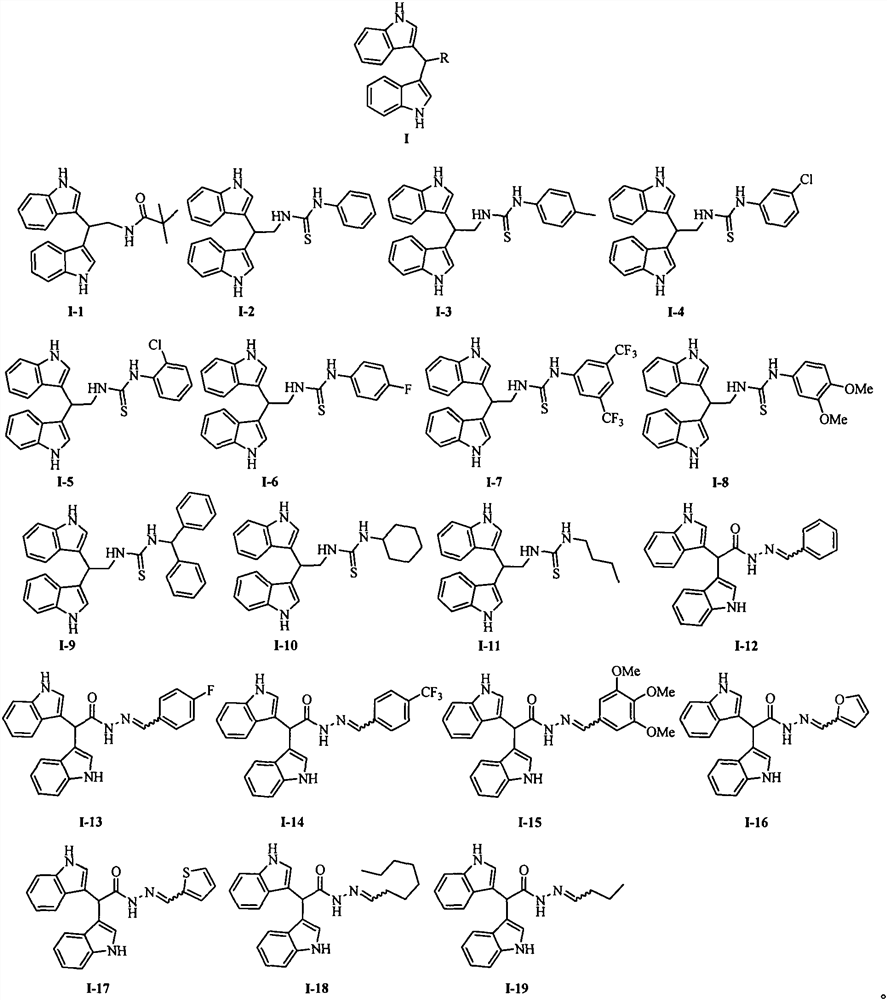
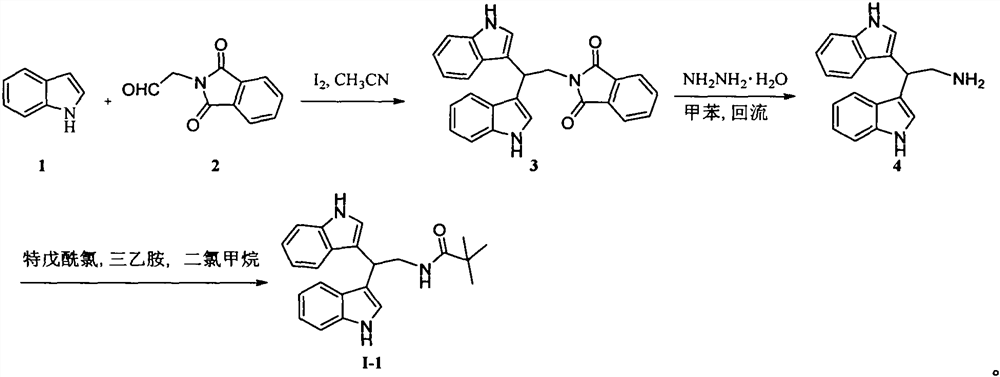
[0022] Example 1: Synthesis of N-(2,2-bis(1H-indol-3-yl)ethyl) pivalamide (I-1):
[0023] The first step: 0 ℃, under stirring state, add I 2 (0.13 g, 0.5 mmol). Keep stirring at this temperature for 30min, add 5% Na 2 S 2 o 3 solution (20 mL) to quench the reaction. The reaction solution was extracted with ethyl acetate (3×50mL), the organic phase was washed with saturated brine (100mL), anhydrous Na 2 SO 4 Dry, filter and concentrate. The residue was subjected to column chromatography with petroleum ether and ethyl acetate (2:1, v / v) to obtain a pink solid compound 2-(2,2-bis(1H-indol-3-yl)ethyl)isoindoline - 1,3-Dione (3) (0.97 g, 96%). Melting point 210-211°C; 1 H NMR (400MHz, CDCl 3 )δ7.97(s, 2H, NH), 7.75(d, J=8.0Hz, 2H, Ar-H), 7.72(dd, J=3.2, 5.5Hz, 2H, Ar-H), 7.61(dd, J=3.0, 5.4Hz, 2H, Ar-H), 7.29(d, J=8.0Hz, 2H, Ar-H), 7.13(t, J=7.2Hz, 2H, Ar-H), 7.09(d, J=2.0Hz, 2H, Ar-H), 7.03(t, J=7.5Hz, 2H, Ar-H), 5.31(t, J=7.7Hz, 1H, Ar 2 -CH), 4.37 (d, J=7.8Hz, 2H, C...
Embodiment 2
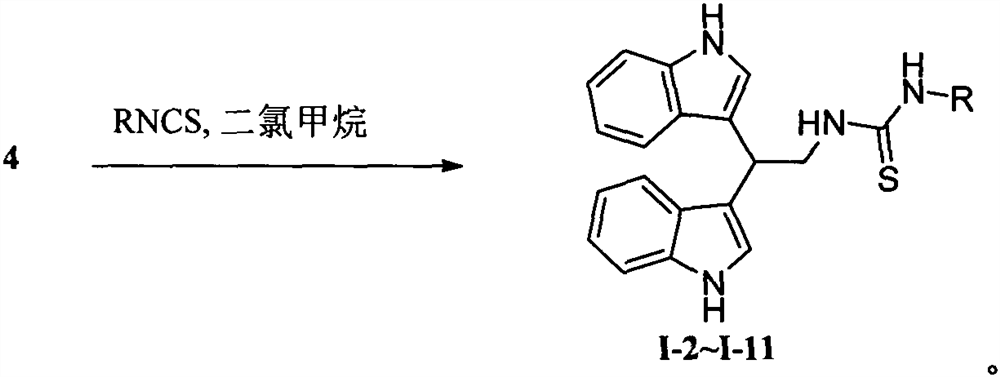
[0026] Embodiment 2: the synthesis of alkaloid streptindole derivatives I-2~I-11:
[0027] 0°C, under stirring, the corresponding isothiocyanate (3.64 mmol) was slowly added to a solution of compound 4 (1 g, 3.64 mmol) in dichloromethane (50 mL), and the reaction solution naturally rose to room temperature, and reacted for 3 h. Slowly add water (40 mL) to the reaction system dropwise to quench the reaction, separate the layers, extract the aqueous phase with dichloromethane (30 mL×2), combine the organic phases with saturated sodium bicarbonate solution (40 mL), saturated saline solution (40 mL ) washing, anhydrous Na 2 SO 4 Drying, suction filtration, concentration, and column chromatography yielded compounds I-2 to I-11;
[0028] 1-(2,2-bis(1H-indol-3-yl)ethyl)-3-phenylthiourea (I-2). Yellow-brown solid; yield 70%; melting point 214-215°C; 1 H NMR (400MHz, CDCl 3 )δ8.00(s, 2H, NH), 7.64(d, J=8.0Hz, 2H, Ar-H), 7.36(t, J=8.4Hz, 4H, Ar-H), 7.29(d, J= 7.0Hz, 1H, Ar-H), 7.18...
Embodiment 3
[0038] Embodiment 3: the synthesis of alkaloid streptindole derivatives I-12~I-19:
[0039] The first step: 0 ℃, under stirring state, add I 2 (0.13 g, 0.5 mmol). Keep stirring at this temperature for 30min, add 5% Na 2 S 2 o 3 solution (20 mL) to quench the reaction. The reaction solution was extracted with ethyl acetate (3×50mL), the organic phase was washed with saturated brine (100mL), anhydrous Na 2 SO 4 Dry, filter and concentrate. The residue was subjected to column chromatography with petroleum ether and ethyl acetate (5:1, v / v) to obtain brown oily compound 6 (1.45 g, 91%), 1 H NMR (400MHz, CDCl 3 )δ8.03(s, 2H, NH), 7.67(d, J=7.9Hz, 2H, Ar-H), 7.30 (t, J=8.1Hz, 2H, Ar-H), 7.23-7.21(m, 2H, Ar-H), 7.14-7.10(m, 2H, Ar-H), 7.00(d, J=2.0Hz, 2H, Ar-H), 5.53(s, 1H, Ar 2 -CH), 4.25(q, J=7.1Hz, 2H, CH 2 ), 1.29(t, J=7.1Hz, 3H, CH 3 ); 13 C NMR (100MHz, CDCl 3 )δ173.7, 136.3, 126.7, 123.5, 122.1, 119.5, 119.3, 113.5, 111.3, 61.2, 40.7, 14.3.
[0040] Step 2: Add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com