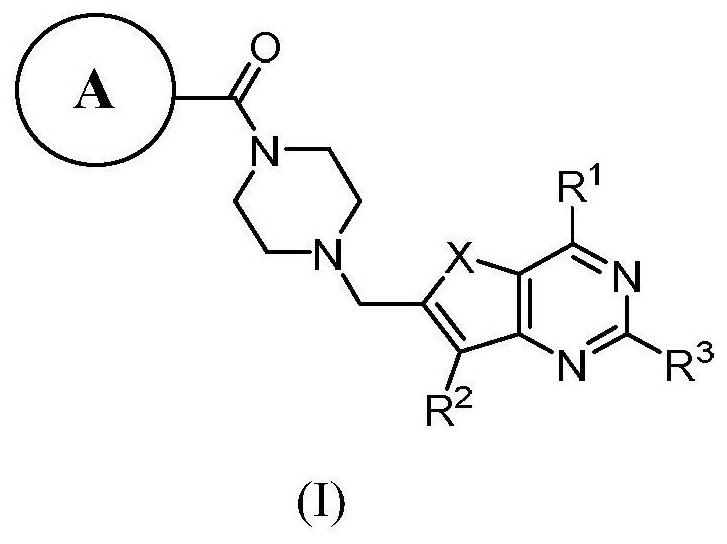
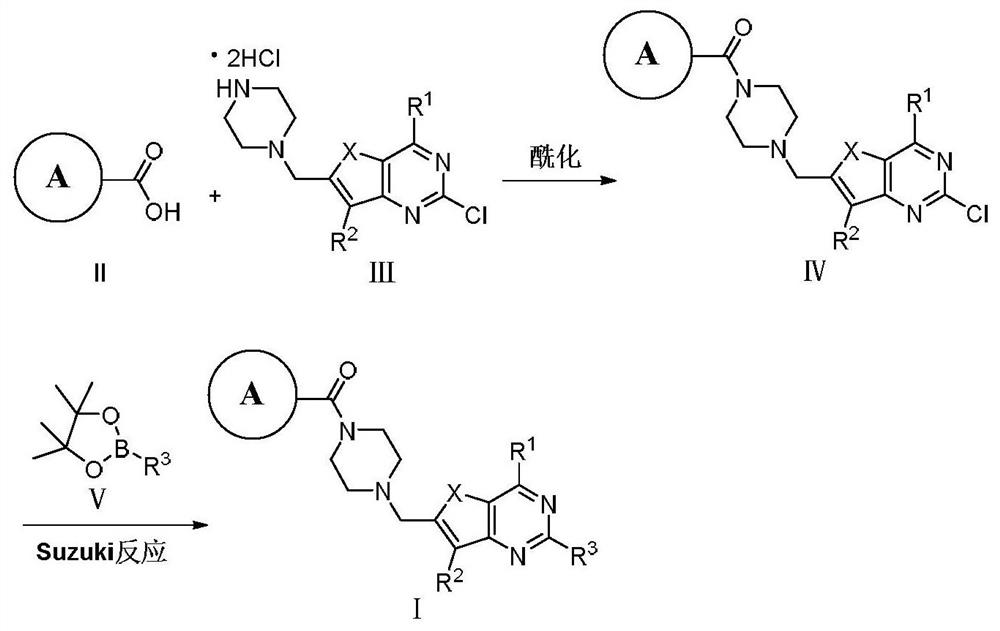
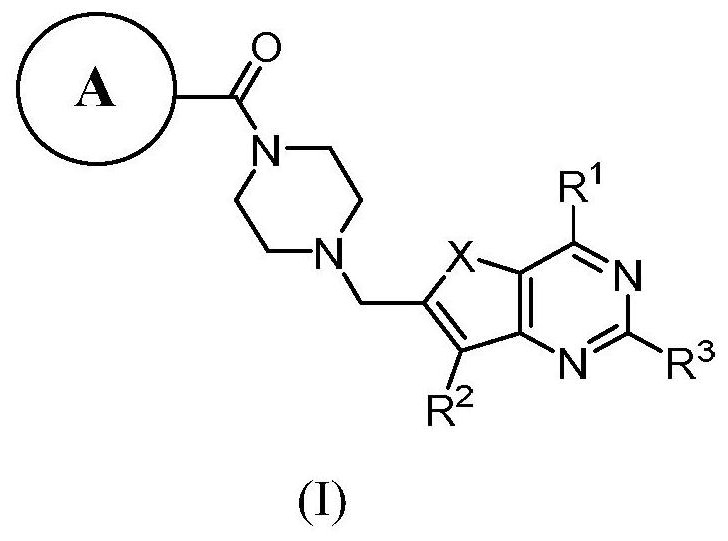
PARP-1/PI3K double-target inhibitor or pharmaceutically acceptable salt thereof, preparation method and application thereof
A PARP-1, dual-target technology, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problem of poor control of dosage, uneven pharmacokinetic properties, drug interaction and side effects, etc. problem, to achieve significant dual inhibitory effects, reduce drug resistance, and reduce drug dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]2-(4-((2-(2-aminopyrimidin-5-yl)-7-methyl-4-morpholine substituted thieno[3,2-d]pyrimidin-6-yl)methyl)piperazine Synthesis of -1-carbonyl)benzofuran-7-carboxamide (I-1)
[0043] 2-(4-((2-chloro-7-methyl-4-morpholine substituted thieno[3,2-d]pyrimidin-6-yl)methyl)piperazine-1-carbonyl)benzofuran- Synthesis of 7-formamide (IV-1)
[0044] Take 4-(2-chloro-7-methyl-6-(piperazin-1-ylmethyl)thieno[3,2-d]pyrimidin-4-yl)morpholine (III-1, 7.00g, 17.00mmol), 7-formamidobenzofuran-2-carboxylic acid (II-1, 3.50g, 17.00mmol), PyBOP (10.70g, 0.021mol) were dissolved in 150mL DMF, slowly dropped into DIEA (11.25mL, 0.068mol), the reaction solution gradually changed from a white turbid solution to a yellow clear solution. The reaction was carried out at 25° C. for 6 h, and the reaction of raw materials was monitored by TLC (dichloromethane:methanol=15:1) to complete the reaction. The reaction was stopped, and 450 mL of water was slowly added at 0°C. A large amount of white solid w...
Embodiment 2
[0052] 2-(4-((2-(6-amino-4-(trifluoromethyl)pyridin-3-yl)-7-methyl-4-morpholine substituted thieno[3,2-d]pyrimidine- Synthesis of 6-yl)methyl)piperazine-1-carbonyl)benzofuran-7-carboxamide (I-2)
[0053] Substitution of 2-(4-((2-chloro-7-methyl-4-morpholine) thieno[3,2-d]pyrimidin-6-yl)methyl)piperazine-1-carbonyl)benzofuran -7-formamide (IV-1, 150mg, 0.27mmol) and 2-amino-4-trifluoromethyl-5-pyridine boronate (V-2, 94mg, 0.32mmol) are raw materials, and the operation process is the same as Synthetic process of compound I-1, 80 mg of white solid was obtained, yield 43.50%. m.p.138-140°C.
[0054] 1 H-NMR (300MHz, DMSO-d 6 )δ (ppm): 8.57 (s, 1H, ArH), 7.89 (d, J=7.7Hz, 1H, ArH), 7.85-7.75 (m, 2H, ArH, CONH 2 ), 7.71(s, 1H, CONH 2 ), 7.50(s, 1H, ArH), 7.42(t, J=7.5Hz, 1H, ArH), 6.85(s, 1H, ArH), 6.80(s, 2H, NH 2 ), 4.00-3.67 (m, 14H, ArCH 2 , CH 2 -O-CH 2 , 2CH 2 -N-CH 2 ), 2.62 (s, 4H, CH 2 -N-CH 2 ), 2.33 (s, 3H, CH 3 ).
[0055] HRMS(ESI):m / z[M+H] + .Calcd fb...
Embodiment 3
[0057] 4-(3-(4-((2-(2-aminopyrimidin-5-yl)-7-methyl-4-morpholine substituted thieno[3,2-d]pyrimidin-6-yl)methyl )Synthesis of piperazine-1-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one (I-3)
[0058] 4-(3-(4-((2-chloro-7-methyl-4-morpholine substituted thieno[3,2-d]pyrimidin-6-yl)methyl)piperazine-1-carbonyl)- Synthesis of 4-fluorobenzyl)phthalazin-1(2H)-one (IV-2)
[0059] With 5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluorobenzoic acid (II-2, 6.77g, 0.023mol) and 4-(2-chloro -7-Methyl-6-(piperazin-1-ylmethyl)thieno[3,2-d]pyrimidin-4-yl)morpholine hydrochloride (III-1, 10.0g, 0.023mol) is The raw material and the operation process were the same as the synthesis process of compound IV-1 to obtain 12.00 g of off-white solid with a yield of 82.50% and m.p.96-98°C.
[0060] 1 H-NMR (300MHz, DMSO-d 6 )δ(ppm): 12.64(s, 1H, CONH), 8.28(d, J=7.9Hz, 1H, ArH), 8.00(s, 1H, ArH), 7.91(t, J=7.6Hz, 1H, ArH ), 7.84(t, J=7.3Hz, 1H, ArH), 7.50-7.42(m, 1H, ArH), 7.36(dd, J=6.3Hz, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com