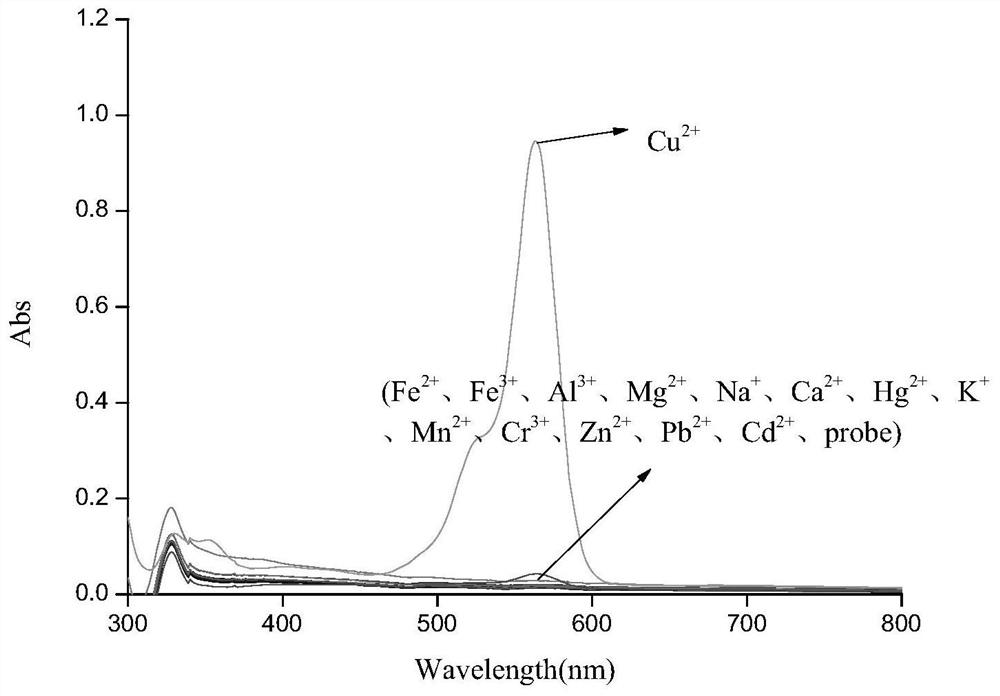
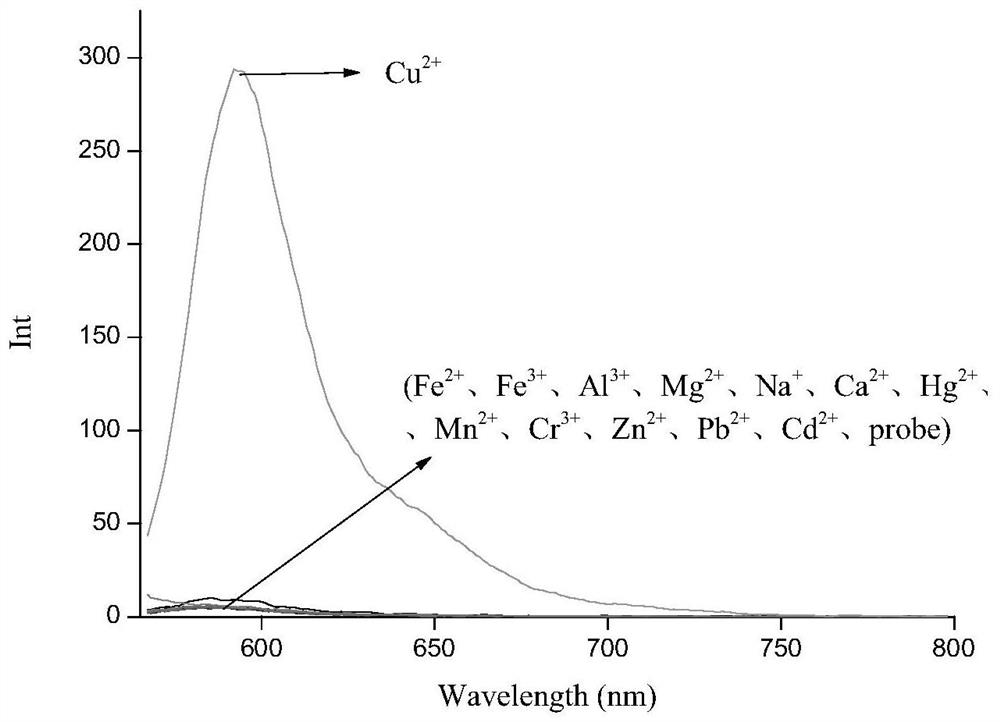
Six-membered spiro rhodamine copper ion fluorescent probe containing hydroxyurea structure as well as preparation method and application of six-membered spiro rhodamine copper ion fluorescent probe
A fluorescent probe and hexa-snail technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of short fluorescence stability time, poor water solubility, strong pH dependence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Six-membered spirocyclic rhodamine copper ion fluorescent probe containing hydroxyurea structure——RhB-OH
[0031] The reaction formula is as follows:
[0032]
[0033] In a round bottom flask, mix 1 mol of Rhodamine B and 3 mol of POCl 3 Add it to dry 120mL of 1,2-dichloroethane, react in an oil bath at 90°C for 4h, cool to room temperature, distill off the solvent under reduced pressure, dissolve the obtained solid in 120mL of dry acetonitrile, add 1.3mol of azide Sodium, stir overnight at room temperature, add anhydrous magnesium sulfate to the reaction solution, dry and filter, heat the filtrate at 82°C for 40 minutes to obtain isocyanate, mix the obtained isocyanate solution with a mixture of 3mol hydroxylamine hydrochloride and 3mol triethylamine, and stir at room temperature, After reacting for 60 minutes, the obtained product was distilled and concentrated under reduced pressure to obtain a six-membered spirocyclic rhodamine copper ion fluorescent p...
Embodiment 2
[0034] Example 2 Six-membered spirocyclic rhodamine copper ion fluorescent probe containing hydroxyurea structure——RhTMR-OH
[0035]
[0036] In a round bottom flask, 1 mol of tetramethylrhodamine TMR and 3 mol of POCl 3 Add it to dry 120mL of 1,2-dichloroethane, react in an oil bath at 90°C for 4h, cool to room temperature, distill off the solvent under reduced pressure, dissolve the obtained solid in 120mL of dry acetonitrile, add 1.3mol of azide Sodium, stir overnight at room temperature, add anhydrous magnesium sulfate to the reaction solution, dry and filter, heat the filtrate at 82°C for 40 minutes to obtain isocyanate, mix the obtained isocyanate solution with a mixture of 3 mol hydroxylamine hydrochloride and 3 mol triethylamine, and react for 60 minutes. After the obtained product is concentrated by distillation under reduced pressure, a six-membered spiro-ring rhodamine copper ion fluorescent probe RhTMR-OH containing a hydroxyurea structure is obtained. HRMS: 41...
Embodiment 3
[0037] Example 3 Six-membered spirocyclic rhodamine copper ion fluorescent probe containing hydroxyurea structure——Rh101-OH
[0038]
[0039] In a round bottom flask, 1 mol of Rhodamine 101 and 3 mol of POCl 3 Add it to dry 120mL of 1,2-dichloroethane, react in an oil bath at 90°C for 4h, cool to room temperature, distill off the solvent under reduced pressure, dissolve the obtained solid in 120mL of dry acetonitrile, add 1.3mol of azide Sodium, stir overnight at room temperature, add anhydrous magnesium sulfate to the reaction solution, dry and filter, heat the filtrate at 82°C for 40 minutes to obtain isocyanate, mix the obtained isocyanate solution with a mixture of 3 mol hydroxylamine hydrochloride and 3 mol triethylamine, and react for 60 minutes. The obtained product was distilled and concentrated under reduced pressure to obtain a six-membered spirocyclic rhodamine copper ion fluorescent probe RH101-OH containing a hydroxyurea structure. HRMS: 520.2474.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com