Culture and identification method for mycobacteria
A technology of mycobacteria and identification method, which is applied in the direction of microorganism-based method, measurement/inspection of microorganism, biochemical equipment and method, etc., and can solve problems such as complicated steps, low number of positive bacteria, and difficulty in identifying mycobacteria , to achieve the effect of tough cell wall, high lipid content, shortening the time of culture and identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] In order to enable those skilled in the art to better understand the technical solutions of the present invention, the present invention will be further described in detail below in conjunction with the accompanying drawings and preferred embodiments.
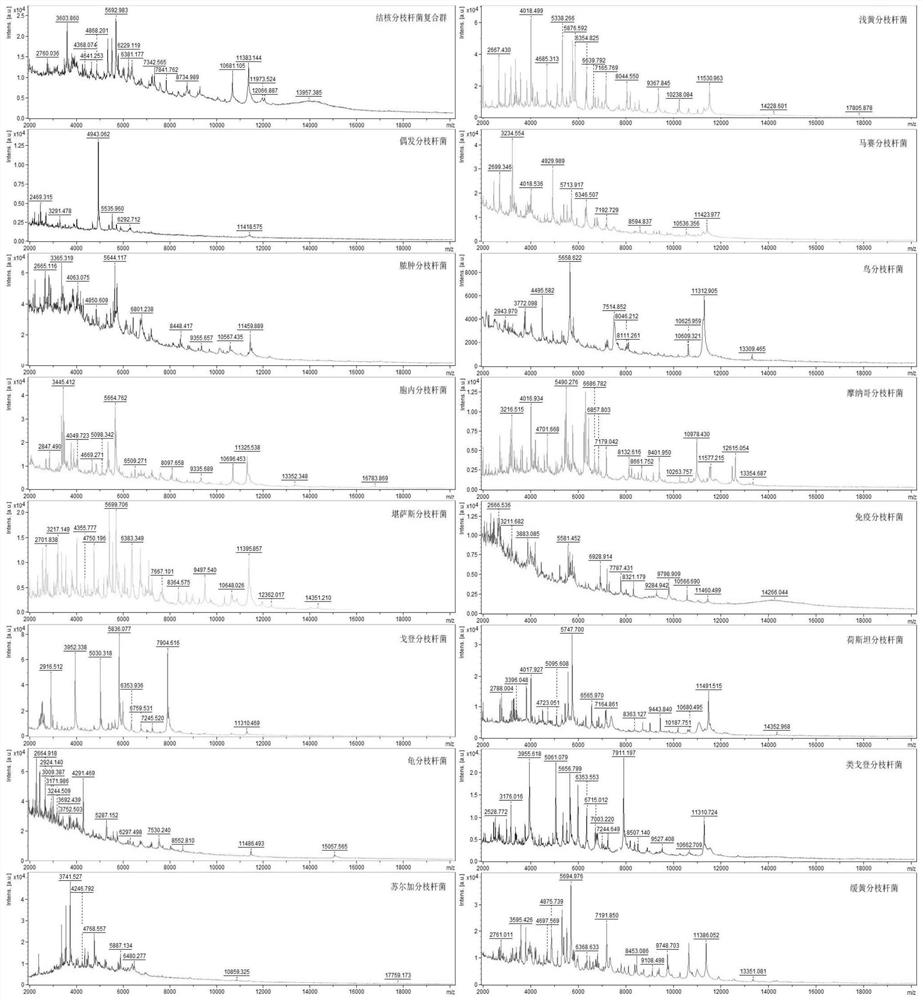
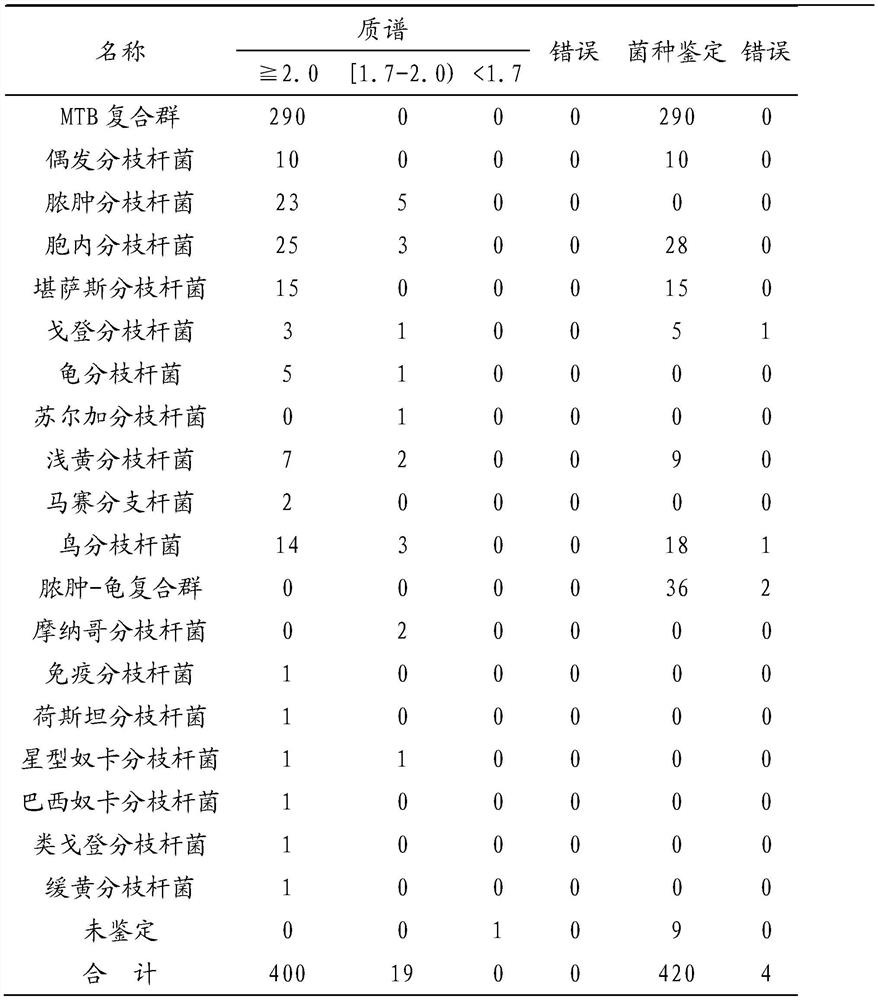
[0032] Research objects: The present invention collects positive cultures from Tianjin Haihe Hospital from January 2019 to December 2019 that were positively reported by the MGIT960 mycobacteria rapid liquid culture instrument, of which 420 cases were used as research objects.
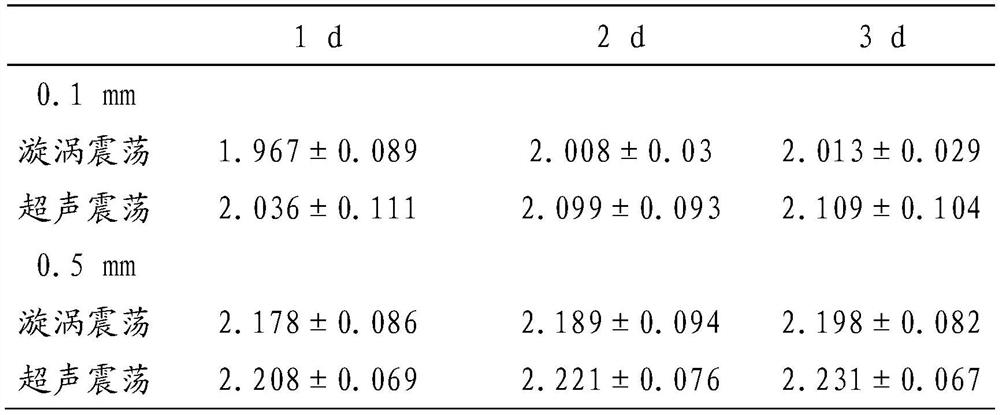
[0033] Reagents and instruments: formic acid, acetonitrile, matrix and standard solvent (Bruker Dahonies, USA), MALDI.TOF target plate and Autoflex MALDI.TOF mass spectrometer (Bruker Dahonies, USA), Flexcontrol3.0 software and Biotyper 3.0 software (Bruker Dahonics, USA company), MALDI Sepsityper Kit (Bruker Dahonics, USA), ultrasonic oscillator, 0.5mm zirconia beads (BioSpec, USA), L-J solid medium (BASO, China), metal bath, mass spectrometry gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com