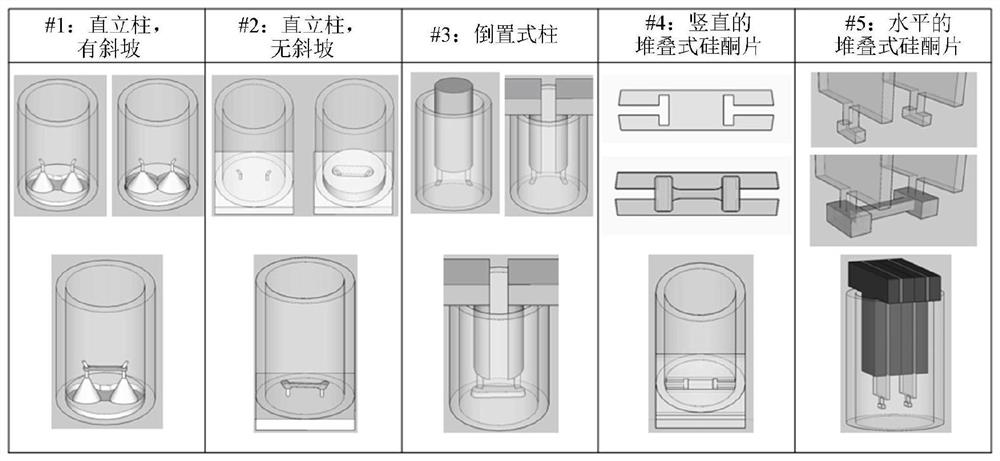
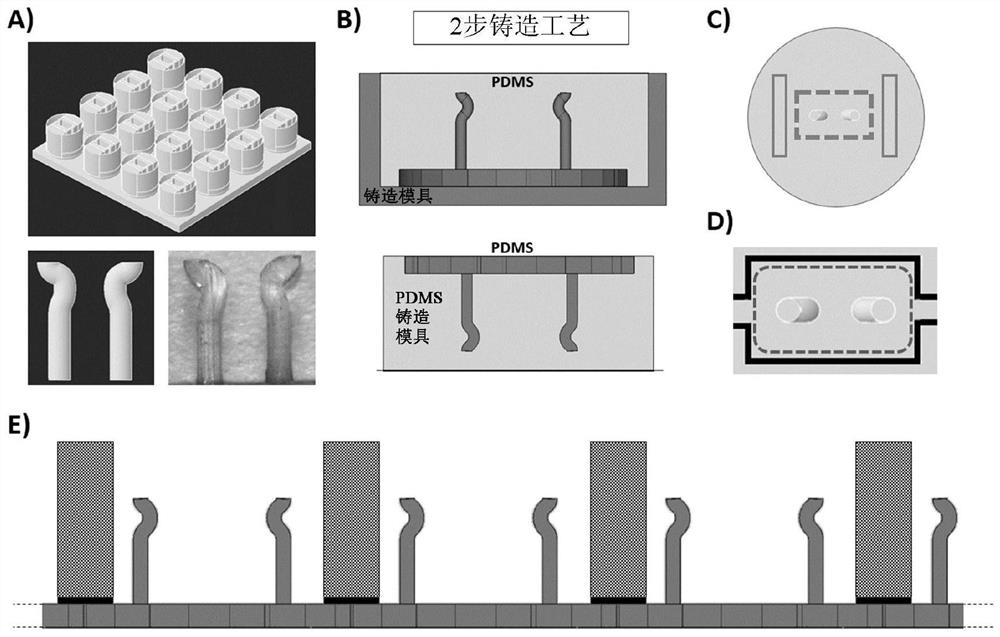
Microfabricated devices and high throughput assays for modulators of cell behavior
A technology for micro-manufacturing and regulators, which can be used in cell culture active agents, enzymology/microbiology devices, embryonic cells, etc., and can solve problems such as low throughput
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0049] method
[0050] Preparation of cardiomyocytes
[0051] The hES2 cell line was used to differentiate human embryonic stem cells (hESCs) into human ventricular-like cardiomyocytes (hvCM) according to Novoheart's proprietary protocol based on the embryoid body approach [8]. Briefly, at 37 °C using 5% CO 2 Cells were maintained on matrigel-coated plates with mTeSR1. On the first day of differentiation, cells were digested to form a suspension in ultra-low attachment plates containing matrigel, 1 ng / ml bone morphogenic protein 4 (BMP4), and 10 μM ROCK inhibitor Y-27632 ( RI) small cell clusters persisting for 24 hours in mTeSR1. The medium was then replaced with StemPro-34 medium with GlutaMAX supplemented with 50 μg / mL ascorbic acid, 10 ng / mL Activin A, 10 ng / mL BMP4 and 5 μM RI. After 3 days, the cells were cultured for 4 days in StemPro-34 medium supplemented with 50 μg / mL ascorbic acid and 5 μM IWR-1. Thereafter, cell clusters were maintained in RPMI 1640 suppleme...
example 2
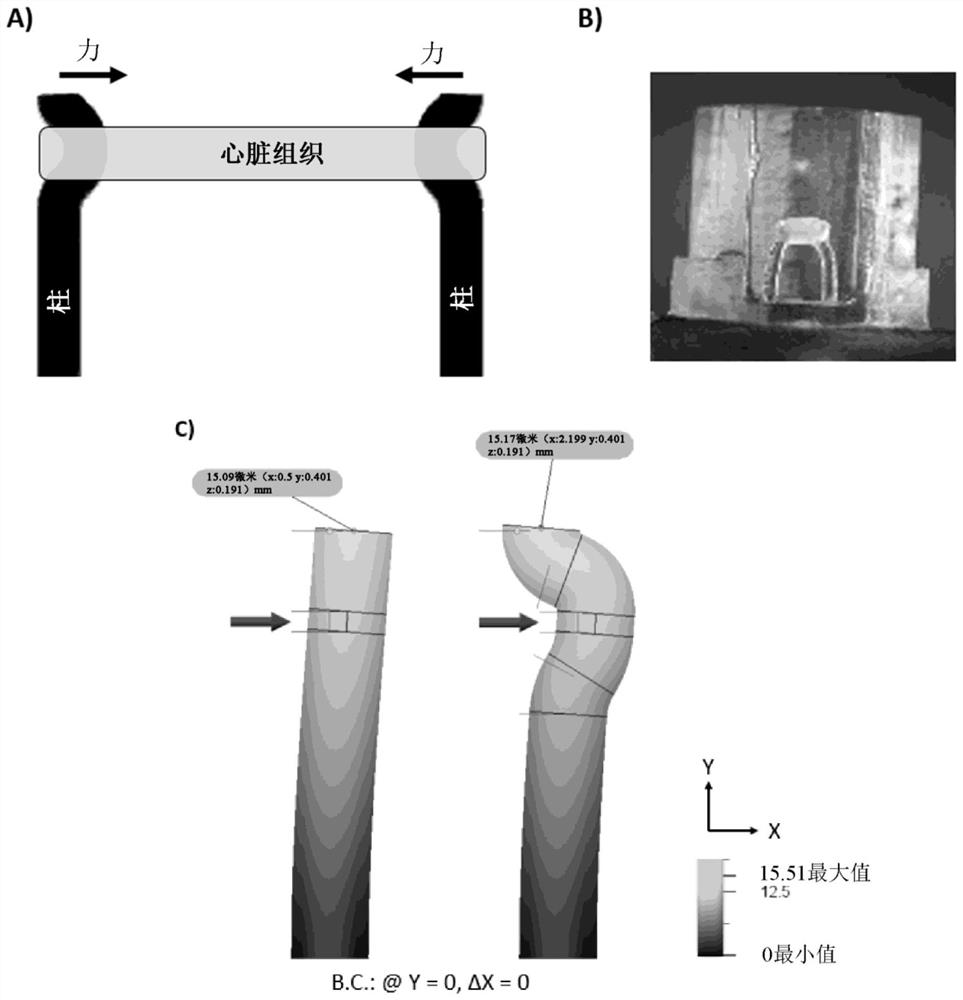
[0064] Force Measurement of Tissue on a Bending Column
[0065] Use bright-field microscopy to image the movement of the column over time due to the beating of the attached tissue strip ( image 3 A). Use custom LabVIEW code to track and record bar positions ( Figure 4 ). The position data was analyzed using a custom MATLAB code utilizing the beam bending equation to estimate the applied force ( Figure 4 )[9]. The beam bending equation is where F is the force, E is the Young's modulus of the column material, R is the radius, a is the height at which the force is applied on the column, L is the full column height, and δ is the displacement of the center of the column. It is estimated that the tissue is anchored at the center of the curved portion of the post. The system was engineered and aimed at centering tissue attachment at the center of the curvature and then visually confirming it afterward using biopsy cultures. Perform finite element modeling to confirm whet...
example 3
[0067] Fabrication and Assembly of Vertical Stacked Cardiac Tissue Culture Plates
[0068] To fabricate planar cardiac tissue prototype plates, a stacking or layering approach ( Figure 6 ), the method consisted of 1) bonding a thin silicone sheet to a commercial bottomless polystyrene cell culture plate, 2) laser cutting the thin silicone sheet in a desired pattern, 3) fabricating a tissue-forming trough layer, and 4) adhering the trough layer to the bottomless plate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com