Methane dry reforming reaction under microwave condition and catalyst thereof
A methane dry reforming and catalyst technology, applied in physical/chemical process catalysts, inorganic chemistry, bulk chemical production, etc., can solve the problem of inability to maintain efficient conversion of methane and carbon dioxide for a long time, high operating costs at reaction temperature, energy loss, etc. problem, to achieve the effect of easy and accurate control of preparation conditions, good catalytic activity and stability, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
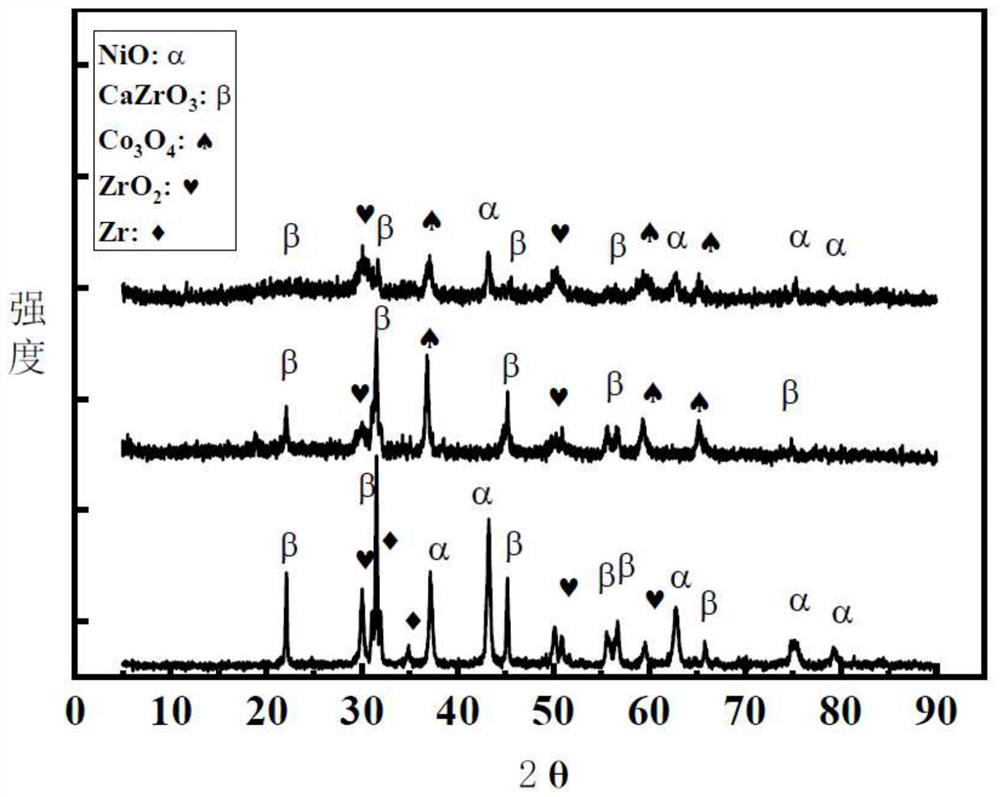
[0033] Preparation of composite metal oxides: Weigh metal salts of zirconium nitrate, calcium nitrate and nickel nitrate according to the formula, dissolve them in distilled water and stir evenly, wherein the ratio of nickel, zirconium and calcium is 2:1:5, Sonicate for 15 minutes, raise the temperature to 50°C, stir for 15 minutes, then raise the temperature to 60°C, stir for 2 hours; add precipitant sodium hydroxide to adjust the pH value to 10 after mixing evenly, stir for 2 hours, let it stand for aging for 2 hours, filter to obtain the filter residue, and The filter residue is dried in an oven at a drying temperature of 120°C, and then the dried filter residue is calcined in a muffle furnace at 700°C for 4 hours to obtain the composite metal oxide Ni / CaZrO 3 .
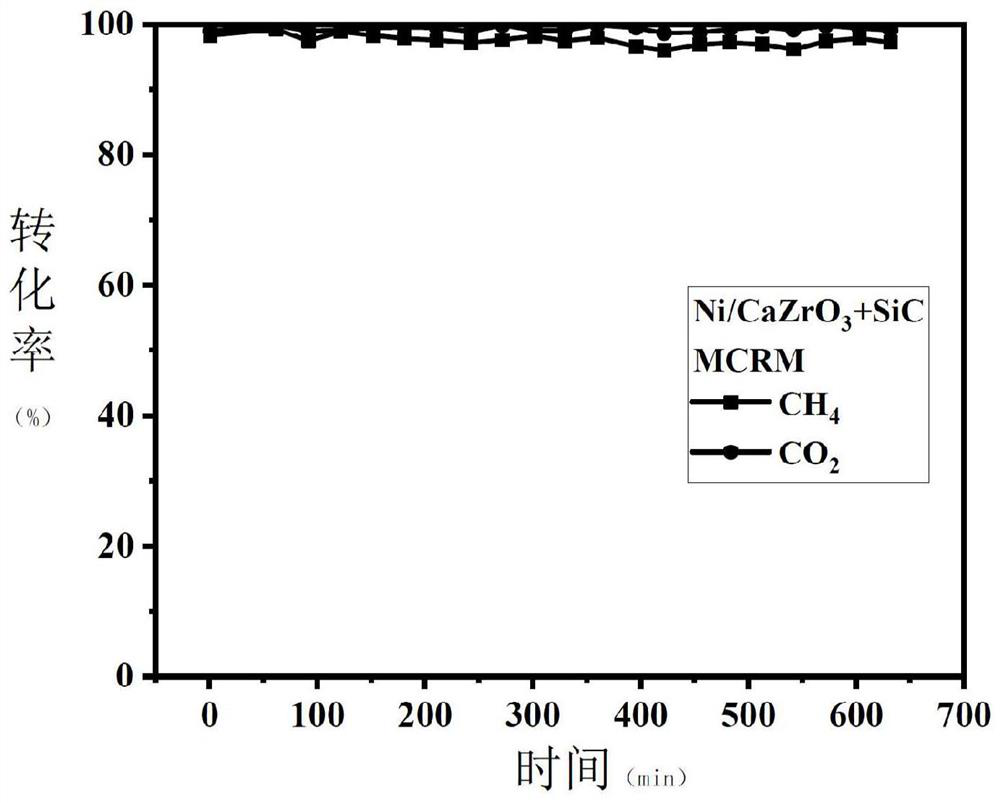
[0034] The preparation of catalyst: take by weighing 1 gram of said composite metal oxide Ni / CaZrO 3 Carry out mechanical mixing with 5 grams of SiC particles; Then the mixed metal oxide Ni / CaZrO 3 A total of 6 ...
Embodiment 2
[0036]Preparation of composite metal oxides: Weigh metal salts of zirconium nitrate, calcium nitrate, and cobalt nitrate, dissolve them in distilled water and stir evenly. minutes, raise the temperature to 50°C, stir for 15 minutes, then raise the temperature to 60°C, and stir for 2 hours; after mixing evenly, add the precipitating agent sodium hydroxide to adjust the pH value to 10, stir for 2 hours, let stand for aging for 2 hours, filter to obtain the filter residue, put the filter residue in Dry in an oven at a drying temperature of 120°C, and then place the dried filter residue in a muffle furnace at 700°C for 4 hours to obtain the composite metal oxide Co / CaZrO 3 .
[0037] Preparation of catalyst: take 1 gram of the composite metal oxide Co / CaZrO 3 Carry out mechanical mixing with 5 grams of SiC particles; Then the mixed metal oxide Co / CaZrO 3 A total of 6 grams of SiC particles were filled into a quartz tube to form a catalyst bed, and Ar gas was introduced to raise ...
Embodiment 3
[0039] Preparation of composite metal oxides: Weigh metal salts of zirconium nitrate, calcium nitrate, cobalt nitrate, and nickel nitrate and dissolve them in distilled water and stir evenly, wherein (the ratio of the amount of nickel element + cobalt element, zirconium element and calcium element is 2 : 1:5, ultrasonic for 15 minutes, heat up to 50°C, stir for 15min, then heat up to 60°C, stir for 2h; mix well, add precipitant sodium hydroxide to adjust the pH value to 10, stir for 2h, let stand for aging for 2 hours, Filtrate to obtain the filter residue, place the filter residue in an oven to dry at 120°C, and then place the dried filter residue in a muffle furnace at 700°C for calcination for 4 hours to obtain the composite metal oxide Ni-Co / CaZrO 3 .
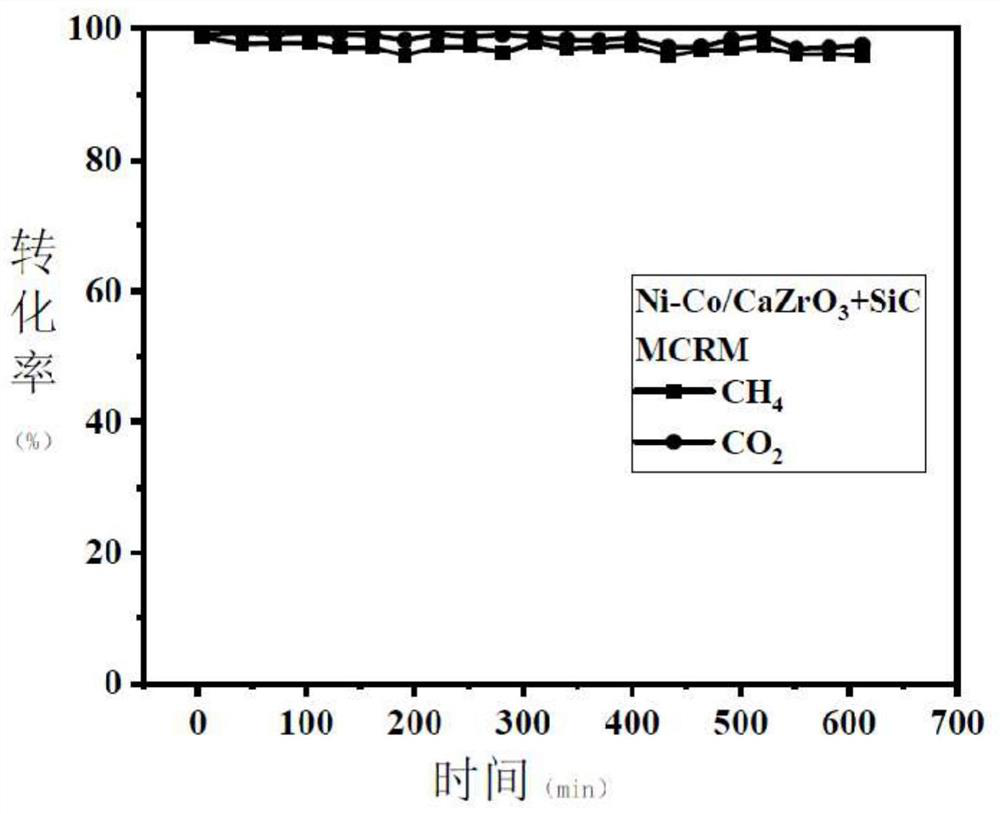
[0040] Catalyst preparation: weigh 1 gram of the composite metal oxide Ni-Co / CaZrO 3 and 5 grams of SiC particles are mechanically mixed; then the mixed metal oxide Ni-Co / CaZrO 3 A total of 6 grams of SiC particles are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com