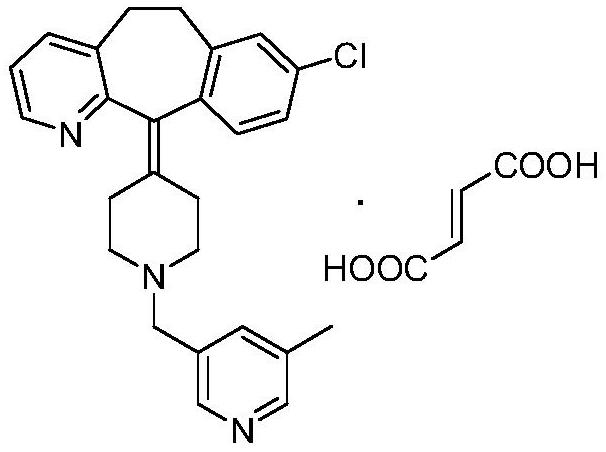
Preparation method of rupatadine fumarate intermediate 5-methyl-3-chloromethylpyridine hydrochloride
A technology of chloromethylpyridine hydrochloride and rupatadine fumarate, applied in the field of synthesis of rupatadine fumarate, can solve the problems of poor product purity, difficult purification, harsh production conditions, etc. It achieves the effects of convenient post-treatment purification, improved product purity, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 49.8g of 5-methyl-3-hydroxymethylpyridine and 300g of dioxane into a 1000mL single-necked flask, add 57.7g of thionyl chloride dropwise at room temperature, and after the dropping, raise the temperature to 110°C and reflux for 2 hours. Concentrate under reduced pressure to remove 40%-50% of the solvent content of the reaction solution, add 100mL n-heptane to the residue, stir and disperse, cool to room temperature for rapid crystallization, filter to collect the precipitate, rinse the filter cake with 20mL n-heptane, and place it in an oven at 60°C dry. A khaki solid particle weighing 64.4 g was obtained, with a yield of 90% and a purity of 99.6%.
Embodiment 2
[0032] Add 100g of 5-methyl-3-hydroxymethylpyridine and 500g of dioxane into a 1000mL one-necked flask, add 100.8g of thionyl chloride dropwise at room temperature, and after the dropping, raise the temperature to 110°C and reflux for 2 hours. Concentrate under reduced pressure to remove 40%-50% of the solvent content of the reaction solution, add 200mL n-heptane to the residue, stir and disperse, cool to room temperature for rapid crystallization, filter to collect the precipitate, rinse the filter cake with 40mL n-heptane, and place it in an oven at 60°C dry. A khaki solid particle was obtained, weighing 132.4 g, with a yield of 92% and a purity of 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com