Tricyclic compound and application thereof
A compound, cycloalkyl technology, applied in the field of chemical medicine, can solve problems such as single structure, and achieve the effect of inhibiting proliferation, good inhibitory effect, and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
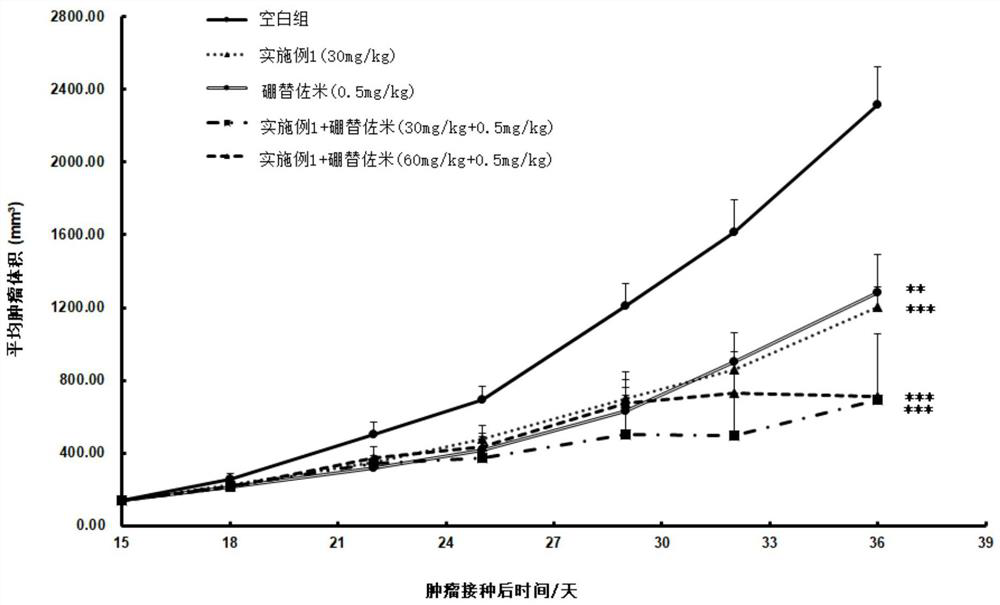
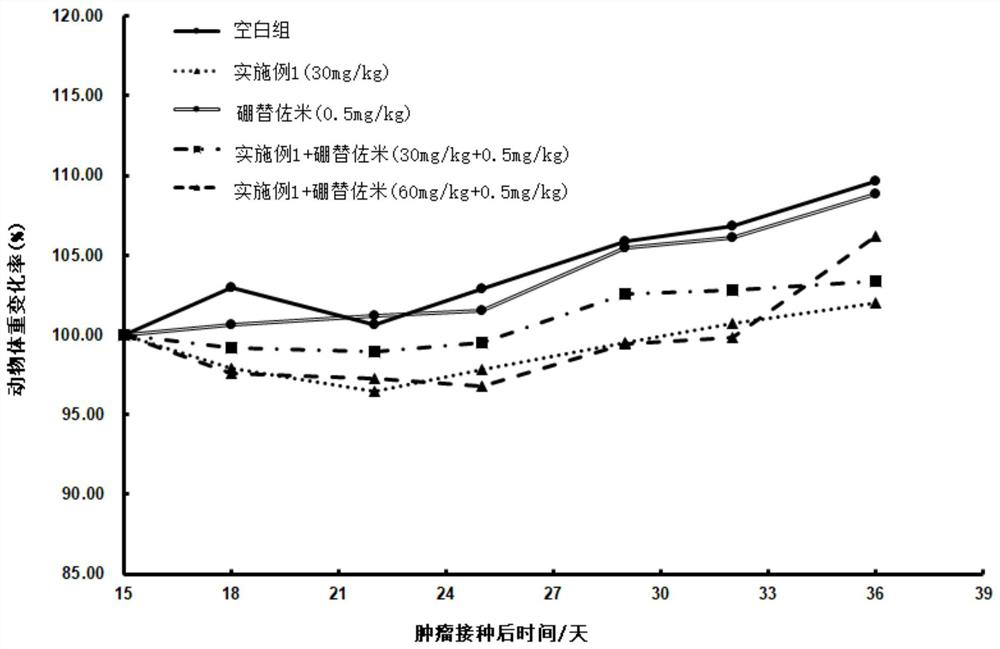
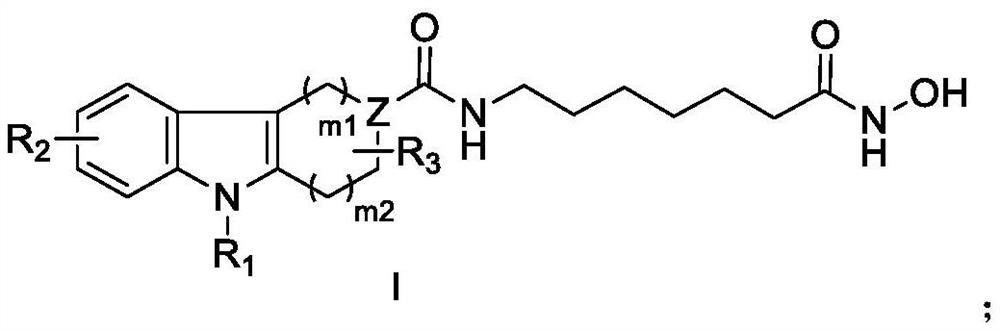
Image
Examples
Embodiment 1
[0117] Example 1: N-(7-(Hydroxyamino)-7-oxoheptyl)-1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole- 2-formamide
[0118]
[0119] Step 1: Preparation of tert-butyl 1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate
[0120] Phenylhydrazine hydrochloride (5.00g, 34.6mmol) and tert-butyl 4-oxopiperidine-1-carboxylate (8.3g, 4.15mmol) were added to acetic acid (50mL), and the reaction mixture was stirred at 65°C to react 16 hours. Concentrate under reduced pressure to remove the organic solvent. Add water (50 mL) to dilute, and extract with ethyl acetate (50 mL×3). The combined organic phases were washed with saturated aqueous sodium bicarbonate (50 mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain a crude product. Separation and purification by column chromatography (silica gel, petroleum ether: ethyl acetate = 10:1-5:1) gave the target compound (5.3 g, yield 56.3%, yellow solid). LC-MS(ESI)m / z[M+H] ...
Embodiment 2
[0127] Example 2: N-(7-(hydroxyamino)-7-oxoheptyl)-5-methyl-1,3,4,5-tetrahydro-2H-pyrido[4,3- b] indole-2-carboxamide
[0128]
[0129] Step 1: Preparation of tert-butyl 5-methyl-1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate
[0130] Dissolve tert-butyl 1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate (500 mg, 1.84 mmol) in N,N-dimethylformamide ( 5 mL), iodomethane (287 mg, 2.02 mmol) and potassium hydroxide (310 mg, 5.52 mmol) were added. The reaction mixture was stirred at 60°C for 16 hours. Water (10 mL) was added to the reaction mixture for dilution, and extracted with ethyl acetate (20 mL×3). The combined organic phases were washed with saturated brine (10 mL×3), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain a crude product. Separation and purification by column chromatography (silica gel, petroleum ether: ethyl acetate = 20:1-5:1) gave the target compound (300 mg, yield 57.0%,...
Embodiment 3
[0137] Example 3: N-(7-(hydroxyamino)-7-oxoheptyl)-5-isopropyl-1,3,4,5-tetrahydro-2H-pyrido[4, 3-b] indole-2-carboxamide
[0138]
[0139] Step 1: Preparation of tert-butyl 5-isopropyl-1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate
[0140] Dissolve tert-butyl 1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate (200 mg, 0.734 mmol) in N,N-dimethylformamide ( 5mL). Sodium hydride (60%, 32.3 mg, 0.807 mmol) was added at 0 °C. The reaction mixture was stirred at 0°C for half an hour and then 2-iodopropane (137 mg, 0.807 mmol) was added. The reaction mixture was stirred at room temperature for 16 hours. The reaction mixture was quenched by adding water (10 mL) at 0°C, extracted with ethyl acetate (20 mL×3). The combined organic phases were washed with saturated brine (10 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain a crude product. The target compound (120 mg, yield 52.0%, colorles...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com