Marine fungus-derived meroterpenoids and application thereof in preparation of anti-inflammatory drugs
A technology of marine fungi and compounds, which is applied in the field of pharmaceutical compounds, can solve problems such as adverse reactions and achieve good results in application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The acquisition of embodiment 1 marine fungus Aspergillus terreus GZU-31-1
[0039] The inventor team isolated a marine fungus, Aspergillus terreus GZU-31-1, from the trunk of the sulphurous back tumor in Xuwen, Zhanjiang City, Guangdong Province. The fungus was identified by DNA amplification and sequencing of the ITS region of the fungus. For specific steps, refer to the literature (Nature protocols, 2010, 5, 480-490; Journal of Natural Products, 2006, 69, 11, 1622-1625): using DNeasy Plant Mini The Kit kit, according to the steps in the manual, extracts DNA, uses ITS as primers for PCR amplification, and the amplified products are sequenced. The sequence obtained by sequencing was searched through the BLAST database for similarity. After comparison, the similarity between the sequence and the Aspergillus terreus fungus sequence was 100%, and the fungus was determined to be the marine fungus Aspergillus terreus GZU-31-1. The strain was deposited in the Guangdong Micr...
Embodiment 2
[0040] The separation of embodiment 2 compound
[0041] A novel heteroterpene derivative was isolated from the fermentation broth of the marine fungus Aspergillus terreus GZU-31-1. The specific method is as follows:
[0042] (1) Seed liquid culture of the fungus Aspergillus terreus GZU-31-1: pick the strain and insert it into the slant, cultivate it at 30°C for 3 days, then insert it into the potato glucose water medium, and cultivate it at 30°C for 3 days to obtain the seed liquid; The composition of base is calculated by weight ratio: glucose 0.3%, yeast extract 0.1%, peptone 0.5%, agar 2.5%, sodium chloride 3%, water 98%; The composition of potato dextrose water medium is calculated by weight ratio: In terms of mass ratio, 20% of potatoes, 0.3% of glucose, 3% of sodium chloride, and water make up to 100%.
[0043] (2) Fermentation culture of the fungus Aspergillus terreus GZU-31-1: transfer the strain in the seed solution into the fermentation medium, and let it stand at 2...
Embodiment 3
[0046] The structural analysis of embodiment 3 compound
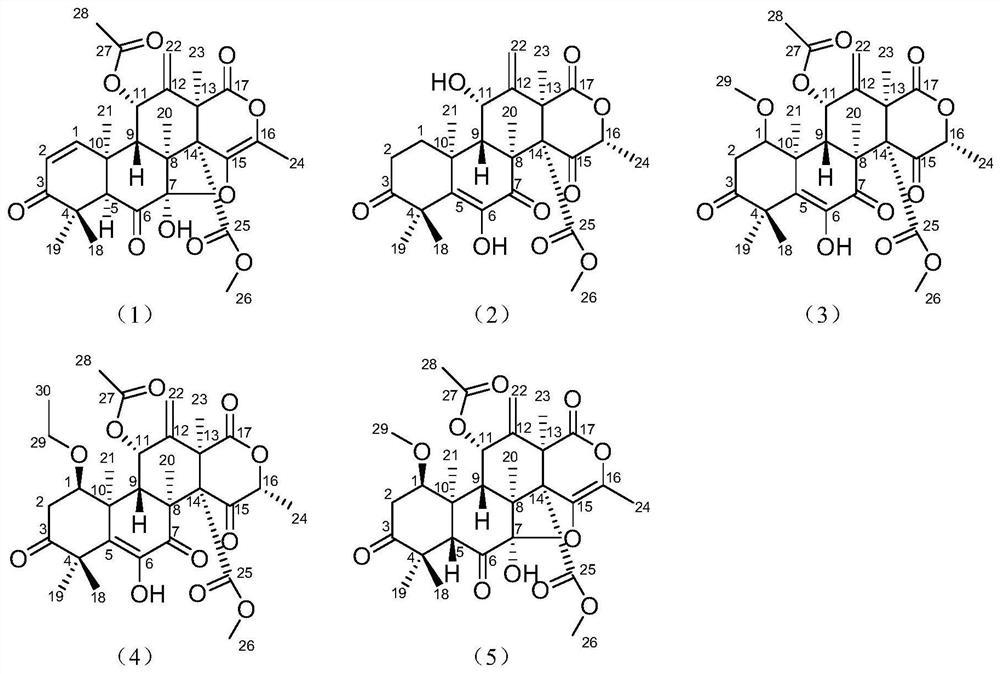
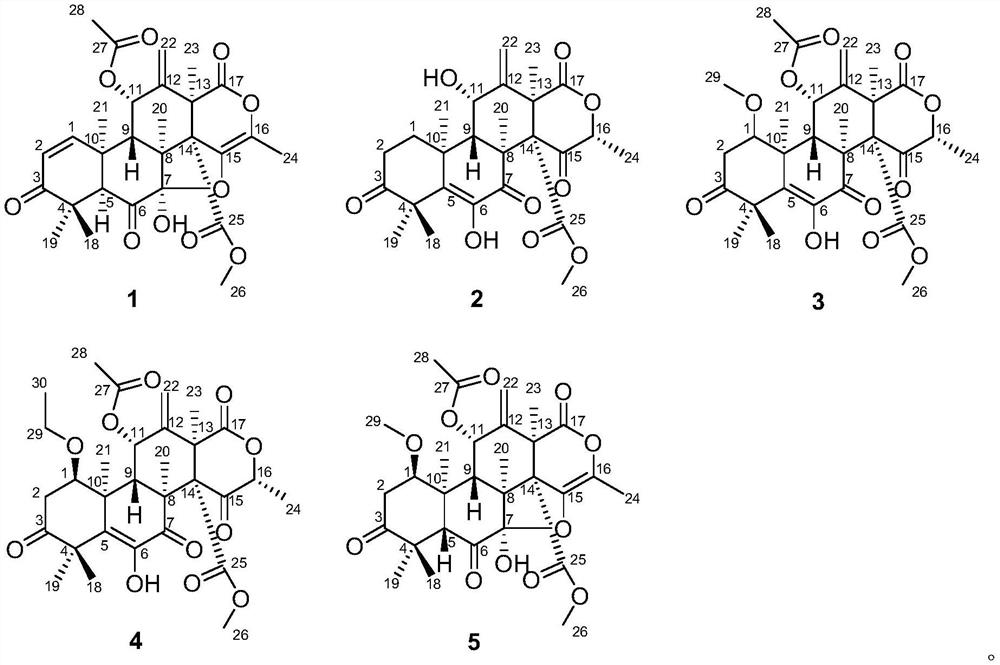
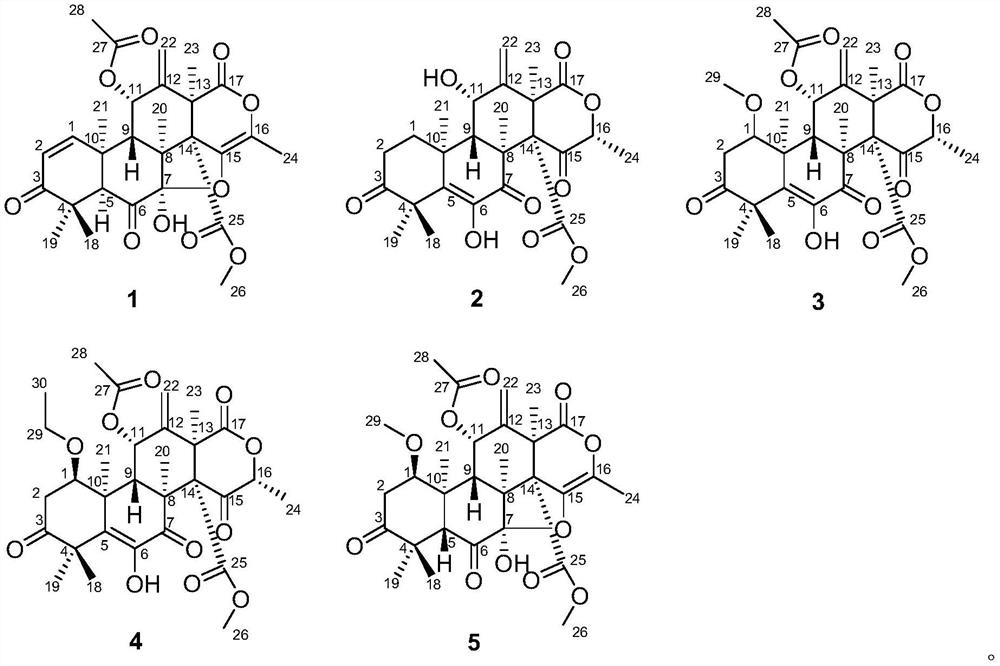
[0047] Structural analysis of new compounds 1-5 was carried out, and the following experimental data were obtained:
[0048] New Compound 1: C 28 h 31 o 10 , HRESI-MS: 527.1915 [M-H] - (calculated value 527.1911).
[0049] New compound 2: C 26 h 31 o 9 , HRESI-MS: 487.1959 [M-H] - (calculated value 487.1962).
[0050] New Compound 3: C 29 h 35 o 11 , HRESI-MS: 559.2183 [M-H] - (calculated value 559.2173).
[0051] New Compound 4: C 30 h 37 o 11 , HRESI-MS: 573.2361 [M-H] - (calculated value 573.2330).
[0052] New Compound 5: C 29 h 35 o 11 , HRESI-MS: 559.2181 [M-H] - (calculated value 559.2173).
[0053] See Table 1 for the NMR data of compounds 1-5.
[0054] The NMR data (CDCl 3 , 400MHz / 101MHz,ppm)
[0055]
[0056]
[0057]
[0058]
[0059] The structural formulas of compounds 1-5 are as follows:
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com