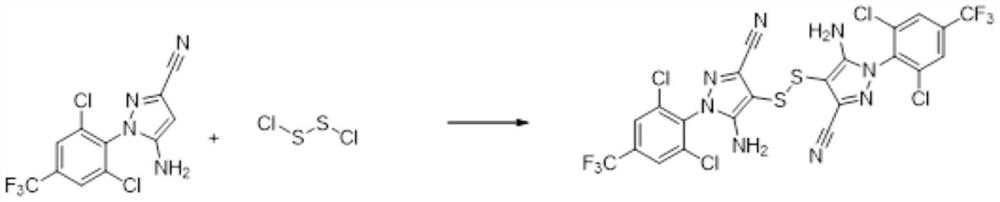
Preparation method of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl) pyrazole disulfide
A technology of trifluoromethylphenyl and trifluoromethyl, which is applied in the field of medicine, can solve the problems of difficult solvent recovery, unfavorable industrial production, and difficult treatment of waste water and liquid, so as to avoid adverse effects, improve production efficiency, The effect of reducing the generation of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
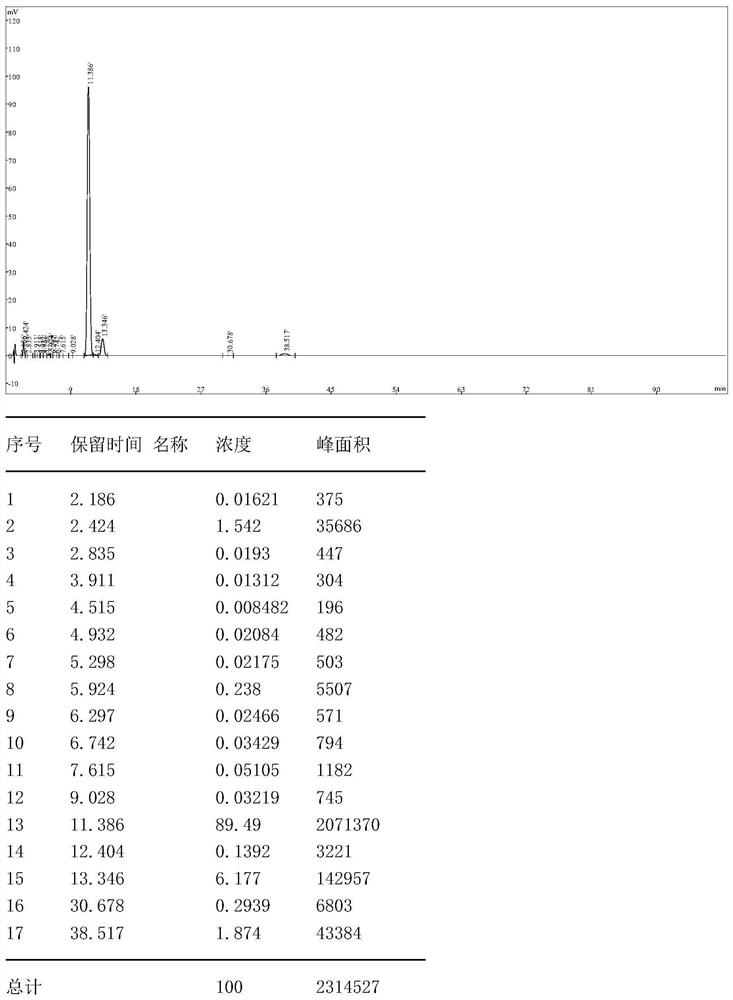
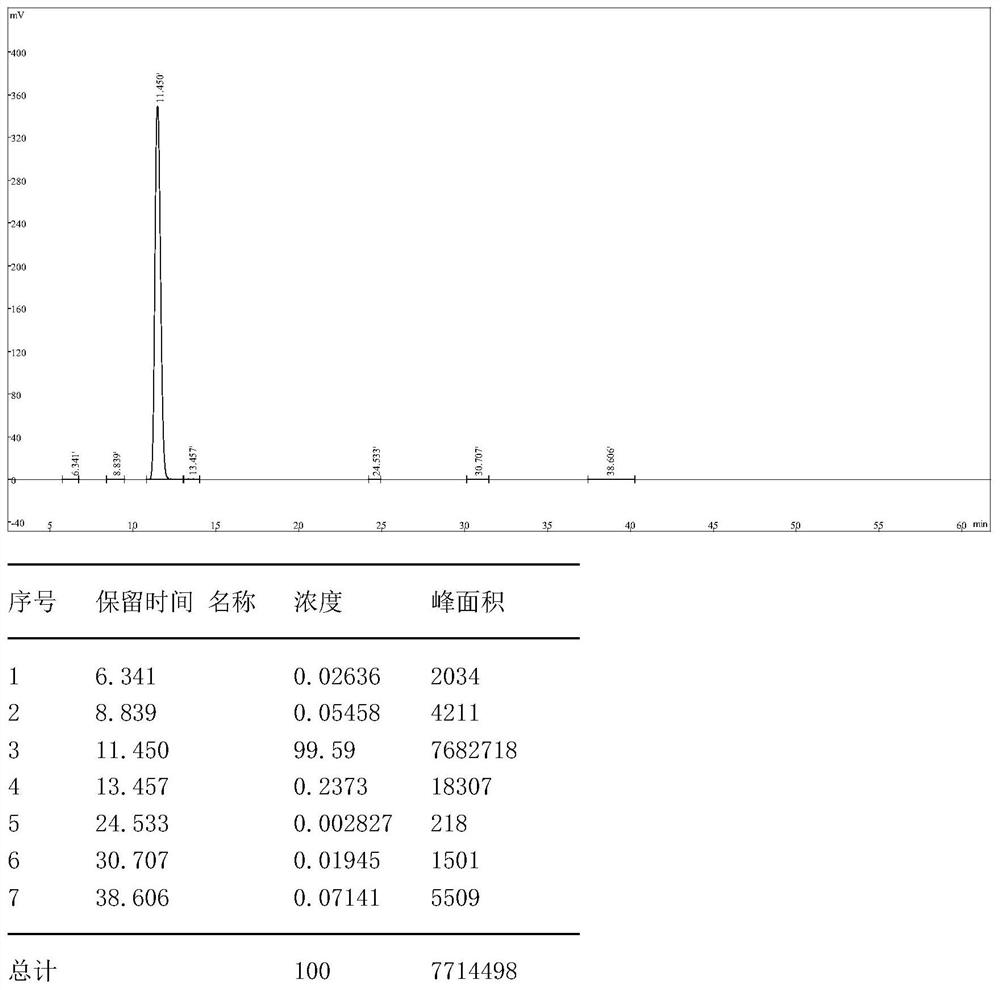
[0067] Step S1: Add a mixed solvent of 300g dichloromethane and 20g tetrahydrofuran to a 500ml three-necked flask, then add 40g 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl ) pyrazole, stir to dissolve completely, cool down to -5~5°C, add 8.54g of sulfur monochloride dropwise, after the dropwise completion, raise the temperature of the reaction solution to 32°C and keep stirring for 2h, in a mixed solvent of dichloromethane and tetrahydrofuran A halogenation reaction occurs to generate 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethyl-phenyl)pyrazole disulfide, and a sample is taken for HPLC to determine that the purity is >80%;
[0068] Step S2: Distill the 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethyl-phenyl)pyrazole disulfide prepared in step S1 under reduced pressure, and distill under reduced pressure The maximum temperature is 60°C, and the vacuum reaches -0.098Mpa. The distillate is a mixed solvent of dichloromethane and tetrahydrofuran. The mixed solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com