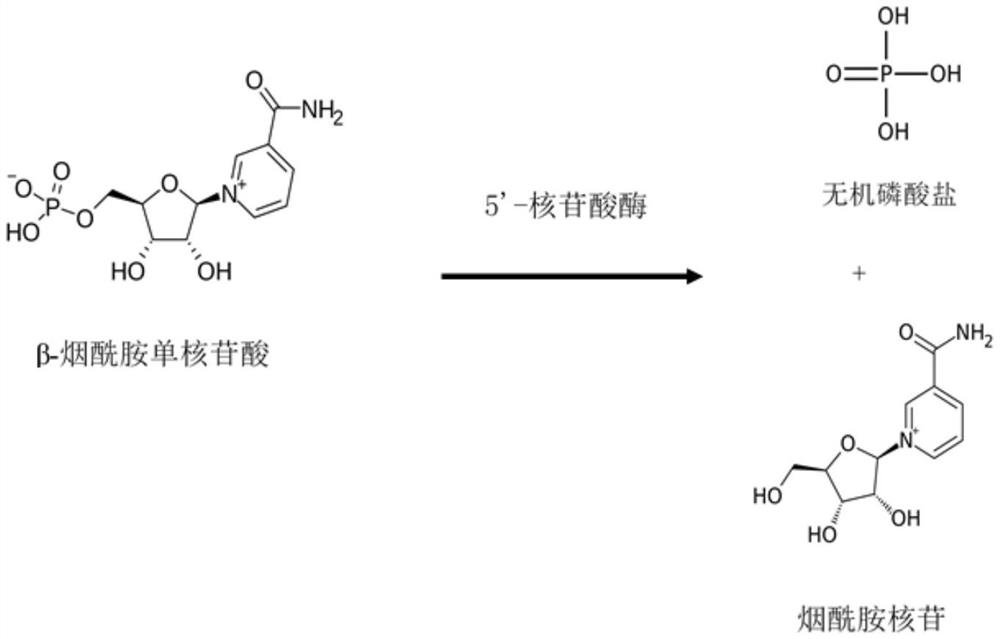
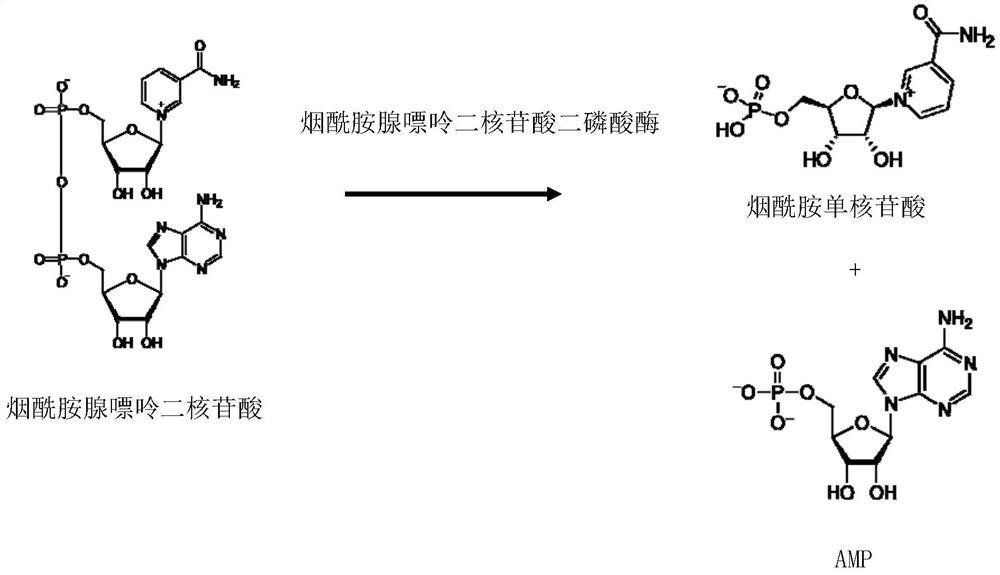
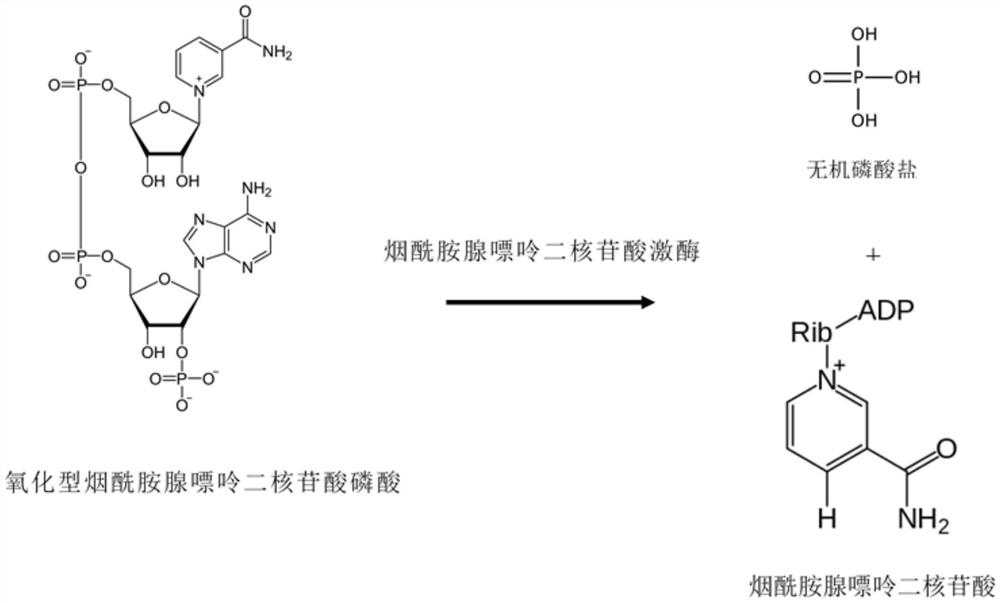
Method for preparing nucleosides of nicotinic acid or derivatives thereof, nicotinic acid adenine dinucleotide and nicotinic acid mononucleotide, enzyme composition and application
A technology of dinucleotide diphosphatase and nicotinic acid adenine, which is applied in the field of biomedicine, can solve problems such as environmental impact, nicotinamide riboside production, and impact, and achieve the effect of improving flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0216] Embodiment 1: Preparation of nicotinamide adenine dinucleotide kinase (EC 2.7.1.23)
[0217] PCR primers were designed according to the DNA sequence (SEQ ID NO.4) (gene bank accession number ARC14363.1) encoding nicotinamide adenine dinucleotide kinase in the Clostridioides difficile genome, specifically:
[0218] Upstream primer NADK1:
[0219] 5'-CTGACC GGATCC ATGAAAAGAATTATAACTATAAAT-3' (SEQ ID NO. 5)
[0220] Downstream primer NADK2:
[0221] 5'-TATGCG GAATTC CTATAAAAAATTTTTCAGATACTCT-3' (SEQ ID NO. 6)
[0222] Clostridioides difficile (Clostridioides difficile) genomic DNA was used as a template, and the above primers were used for PCR amplification to obtain the nicotinamide adenine dinucleotide kinase gene, and the PCR product was treated with restriction endonucleases BamH I and EcoRI and ligated into pET-21a to obtain pET-NADK. The recombinant expression vector is transformed into Escherichia coli HB101 to obtain a recombinant expression strain of nicoti...
Embodiment 2
[0224] Embodiment 2: Preparation of nicotinamide adenine dinucleotide diphosphatase (EC 3.6.1.22)
[0225] PCR primers were designed based on the DNA sequence (PJP11175.1) (SEQ ID NO.7) encoding nicotinamide adenine dinucleotide diphosphatase in the Saccharomyces cerevisiae genome, specifically:
[0226] Upstream primer NDP1:
[0227] 5'-CTGACC GGATCC ATGTCCACTGCAGTGACTTTTTTT-3' (SEQ ID NO. 8)
[0228] Downstream primer NDP2:
[0229] 5'-TATGCG GAATTC CTATAGATGGCTCGATGAGGTCTT-3' (SEQ ID NO. 9)
[0230] Genomic DNA of Saccharomyces cerevisiae was used as a substrate, and the above primers were used to perform PCR amplification to obtain the nicotinamide adenine dinucleotide dinucleotide diphosphatase gene, and the PCR product was treated with restriction endonucleases BamH I and EcoR I and This was ligated into pET-21a to obtain pET-NDP. The recombinant expression vector was transformed into Escherichia coli HB101 to obtain a recombinant expression strain of nicotinamide...
Embodiment 3
[0232] Embodiment 3: Preparation of 5'-nucleotidase (EC 3.1.3.5)
[0233] PCR primers were designed based on the DNA sequence (AVB07708.1) (SEQ ID NO.10) encoding 5'-nucleotidase in the Salmonella enterica genome, specifically
[0234] Upstream primer NUCL1:
[0235] 5'-CTGACC GGATCC ATGAAAGTAAAACTGCTTGCTGCC-3' (SEQ ID NO. 11)
[0236] Downstream primer NUCL2:
[0237] 5'-TATGCG GAATTC TTACTTCTTCACATCCGCAACGCG-3' (SEQ ID NO. 12)
[0238] Using the genomic DNA of Salmonella enterica as a substrate, the 5'-nucleotidase gene was amplified by PCR with the above primers, and the PCR product was treated with restriction endonucleases BamH I and EcoR I and ligated into pET-21a to obtain pET-USHA. The recombinant expression vector was transformed into Escherichia coli HB101 to obtain a 5'-nucleotidase recombinant expression strain.
[0239] Select a single colony of the above strains and inoculate them into 4 mL of LB medium (containing 100 μg / ml ampicillin), and culture them ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com