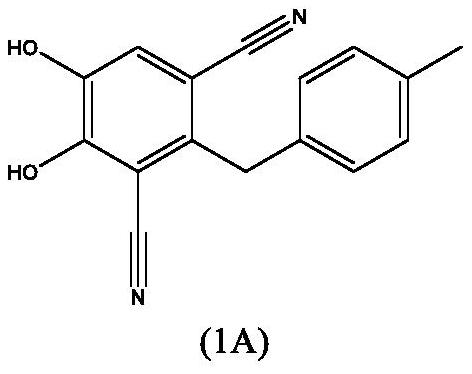
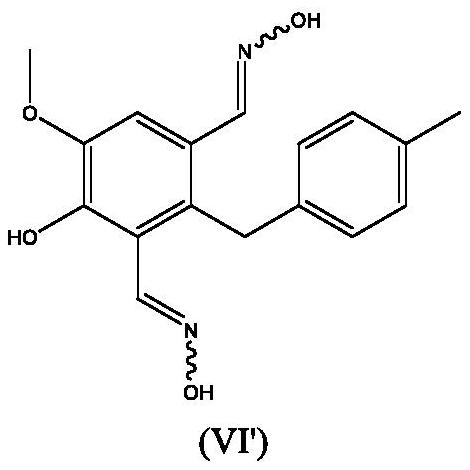
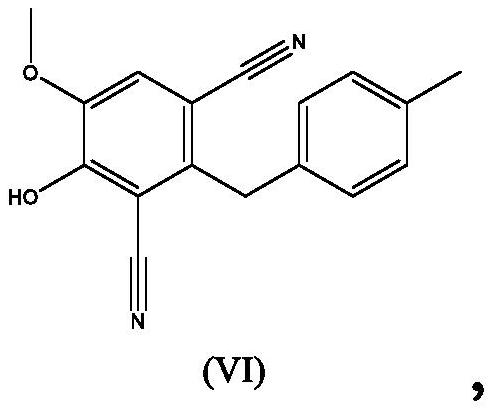
Process for the preparation of 4,5-dihydroxy-2-(4-methylbenzyl)isophthalonitrile
A technology of methyl benzyl and m-phthalocyanine, which is applied in the preparation of oxime, carboxylic acid nitrile, and carboxylate, and can solve the problems of limited commercial availability and difficult recovery of palladium catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Embodiment 1: Preparation of 2-methoxyphenyl 2-chloroacetate
[0111] 2-Methoxyphenol (20 mL), dichloromethane (60 mL) and water (28 mL) were added. 50% NaOH (8.0 mL) was slowly added at 0-10 °C. 2-Chloroacetyl chloride (10.0 mL) in dichloromethane (20 mL) was slowly added at 0-10 °C. 50% NaOH (7.8 mL) was added at 0-10 °C. 2-Chloroacetyl chloride (9.0 mL) in dichloromethane (10 mL) was slowly added at 0-10 °C. The mixture was stirred at 0-10°C for about 1 hour. 30% HCl (6 mL) and water (60 mL) were added at 0-10°C. The aqueous phase was separated. The organic phase was washed with water (60 mL), and 60 mL of dichloromethane was distilled off. Dichloromethane (100 mL) was added. 60 mL of dichloromethane was distilled off. The solution was used directly in the next step.
Embodiment 2
[0112] Embodiment 2: Preparation of 2-methoxyl-5-(4-methylbenzoyl)phenyl 2-chloroacetate
[0113] Dichloromethane (60 mL) and aluminum chloride (14.8 g) were added. 4-Methylbenzoyl chloride (16 mL) was slowly added at 0-10°C. Half of the solution obtained in Example 1 was slowly added at room temperature. The mixture was stirred overnight. Water (70 mL) and 30% HCl (16 mL) were slowly added at 0-10 °C. The aqueous phase was separated. The solution was used directly in the next step.
Embodiment 3
[0114] Embodiment 3: Preparation (3-hydroxyl-4-methoxyphenyl) (p-tolyl) ketone
[0115] From the solution obtained in Example 2, 50 mL of dichloromethane was distilled off. Methanol (132 mL) and 30% HCl (4.0 mL) were added. About 48 mL distilled off. The mixture was refluxed for 2 hours, then cooled to 0-5 °C. The compound was filtered, washed with methanol (30 mL), and dried under reduced pressure at 50-60 °C. The yield was 85.5% and the HPLC purity was 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com