Sulfur-modified chloroprene rubber and method for producing same, sulfur-modified chloroprene rubber composition, vulcanizate, and molded article
A chloroprene rubber and a manufacturing method technology are applied in the fields of sulfur-modified chloroprene rubber and its manufacture, sulfur-modified chloroprene rubber compositions, sulfides and molded products, and can solve the problem of rubber heating, deterioration, shortening of product life, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
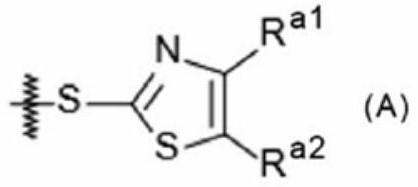
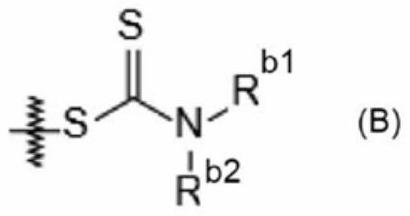
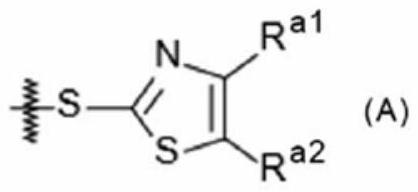
Image
Examples
Embodiment 1
[0109] 100 parts by mass of chloroprene, 0.55 parts by mass of sulfur, 120 parts by mass of pure water, 4.00 parts by mass of disproportionated potassium abietate (manufactured by HARIMA CHEMICALS, INC.), and 0.60 parts by mass of sodium hydroxide were added to a polymerization tank with an inner volume of 30 L. , and 0.6 parts by mass of sodium salt of β-naphthalenesulfonic acid formaldehyde condensate (trade name "DEMOL N": manufactured by Kao Corporation). The pH of the aqueous emulsion before the start of polymerization was 12.8. After adding 0.1 parts by mass of potassium persulfate as a polymerization initiator, emulsion polymerization was performed at a polymerization temperature of 40° C. under a nitrogen stream. When the conversion ratio became 85%, 0.05 parts by mass of diethylhydroxylamine was added as a polymerization terminator to terminate the polymerization, thereby obtaining a polymerization solution of chloroprene.
[0110] To the obtained polymerization solu...
Embodiment 2
[0122] The amount of N-cyclohexyl-2-benzothiazole sulfenamide as a plasticizer was changed from 1 part by mass to 0.5 parts by mass, and the amount of tetrabenzylthiuram disulfide was changed from 4 parts by mass to Raw rubber was obtained by the same method as in Example 1 except that 8 parts by mass.
Embodiment 3
[0124] The amount of N-cyclohexyl-2-benzothiazole sulfenamide as a plasticizer was changed from 1 part by mass to 2 parts by mass, and the amount of tetrabenzylthiuram disulfide added was changed from 4 parts by mass to Raw rubber was obtained by the same method as in Example 1 except that 2 parts by mass.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com