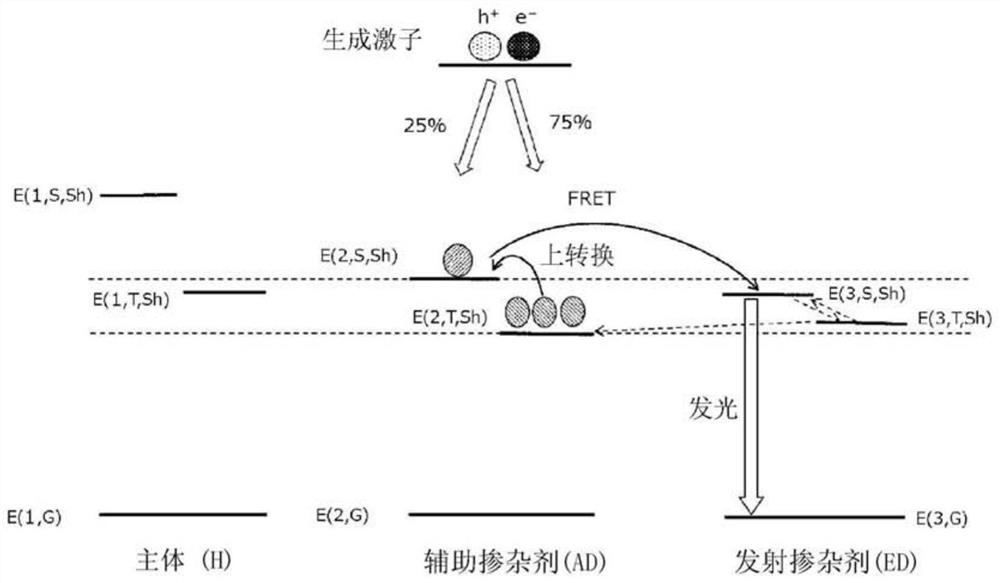
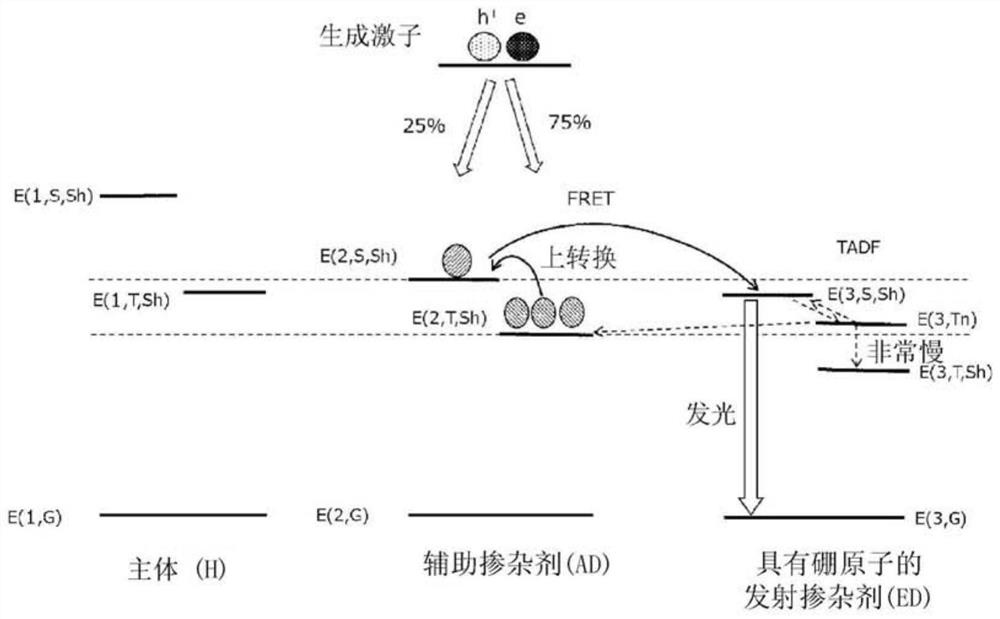
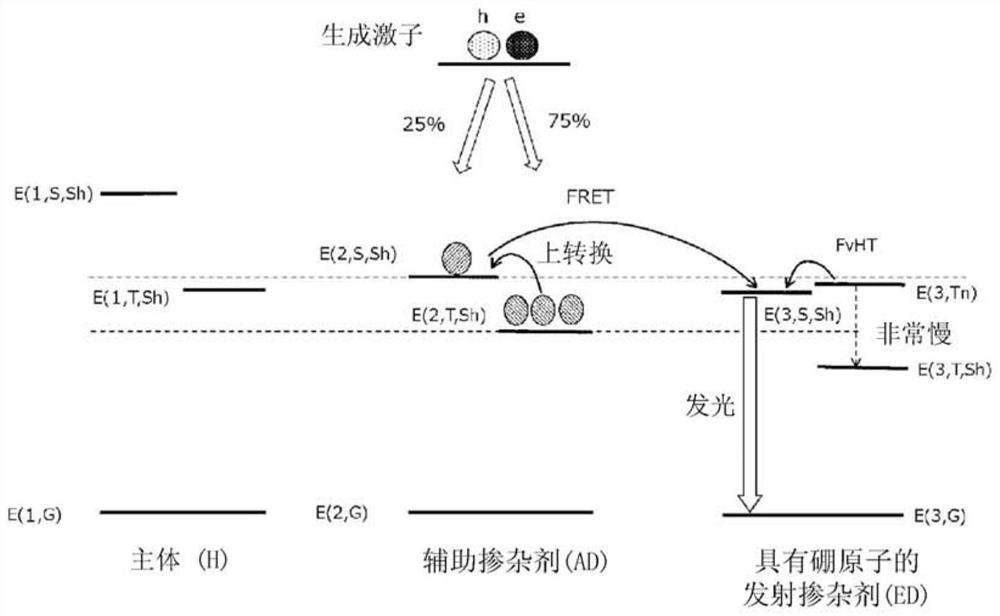
Organic electroluminescent element, display device, illumination device, luminescent layer forming composition, and compound
A technology for electroluminescent elements and light-emitting layers, applied in the fields of compositions and compounds for forming light-emitting layers, display devices and lighting devices, can solve the problem of high energy, efficiency, color purity and lifetime of emission dopants and auxiliary dopants There are problems such as problems, to achieve the effect of improving the characteristics of organic electroluminescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0929] Hereinafter, the present invention will be specifically described through examples. Materials, processing contents, processing procedures, and the like shown below can be appropriately changed unless departing from the spirit of the present invention. Therefore, the scope of the present invention should not be limitedly interpreted by the specific examples shown below.
[0930] Synthesis examples of compounds used in Examples are shown below.
[0931] 1. Compound Synthesis
Synthetic example (1
[0933] Compound (ED1): N 7 ,N 7 ,N 13 ,N 13 ,5,9,11,15-octaphenyl-5,9,11,15-tetrahydro-5,9,11,15-tetraaza-19b,20b-diborabinaphtho[3,2 Synthesis of ,1-de:1',2',3'-jk]pentacene-7,13-diamine
[0934] [chem 161]
[0935]
[0936] [The first stage]
[0937] Under a nitrogen environment, put 1,3-dibromobenzene (25.0g, 106mmol), aniline (20.3ml, 223mmol), tris(dibenzylideneacetone)dipalladium(0)(Pd 2 (dba) 3 ) (971mg, 1.06mmol), 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl (BINAP: 1.98g, 3.18mmol), NaOtBu (25.5g, 265mmol) and toluene (400ml ) flask was heated to 110°C and stirred for 18 hours. The reaction liquid was cooled to room temperature, filtered using silica gel (eluent: toluene), and the solvent was distilled off under reduced pressure to obtain a crude product. After dissolving the obtained crude product in toluene, an appropriate amount was distilled off under reduced pressure, hexane was added and reprecipitated, thereby obtaining N 1 ,N 3 - Diphenylbenzene-1...
Synthetic example (2
[0962] Compound (1-41): 2,12-di-tert-butyl-5,9-bis(4-(tert-butyl)phenyl)-7-methyl-5,9-dihydro-5,9- Synthesis of diaza-13b-boranaphtho[3,2,1-de]anthracene
[0963] [chem 166]
[0964]
[0965] Compound (1-41) was synthesized according to the method described in "Synthesis Example (32)" of International Publication No. 2015 / 102118.
[0966] Hereinafter, in Synthesis Example (1-2) to Synthesis Example (1-4), Synthesis Example (1-9) to Synthesis Example (1-14), the same method as that of Synthesis Example (1-1) was used, Synthesize each compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com