Method for recycling valuable metals of waste lithium ion batteries
A lithium-ion battery and valuable metal technology, which is applied in the field of recycling valuable metals in waste lithium-ion batteries, can solve the problems of reduced work efficiency, increased labor and material costs, and increased surface tension in the process flow, so as to improve the probability of contact reaction and reduce Input of heat source, effect of improving recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
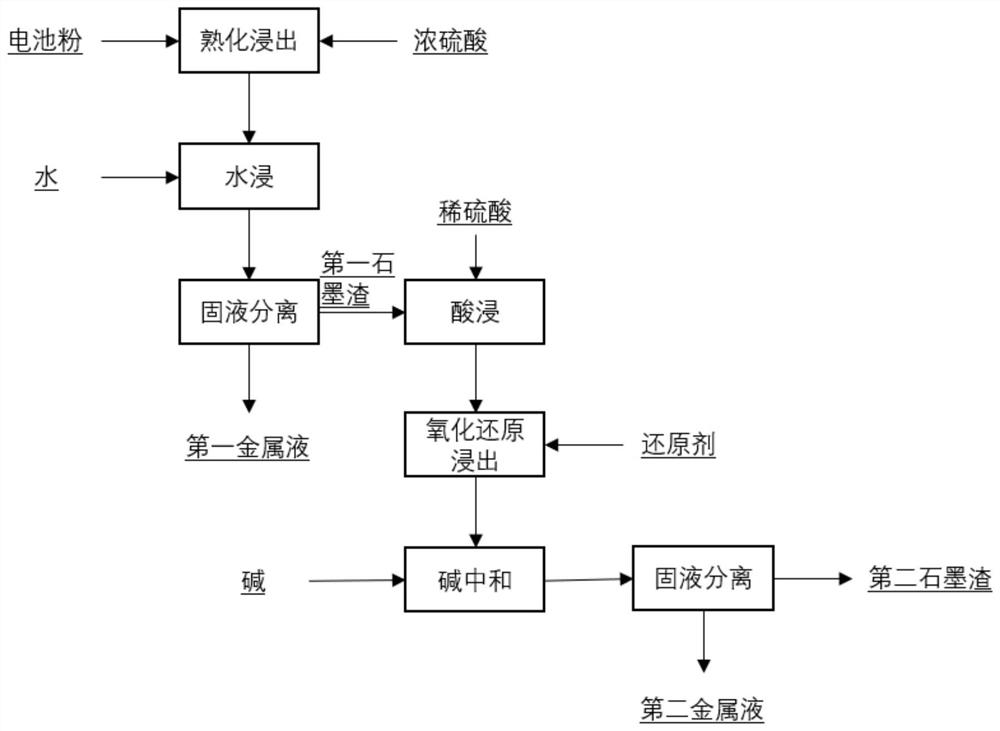
[0039] This embodiment reclaims the valuable metals in the waste lithium ion battery, and the specific process is:
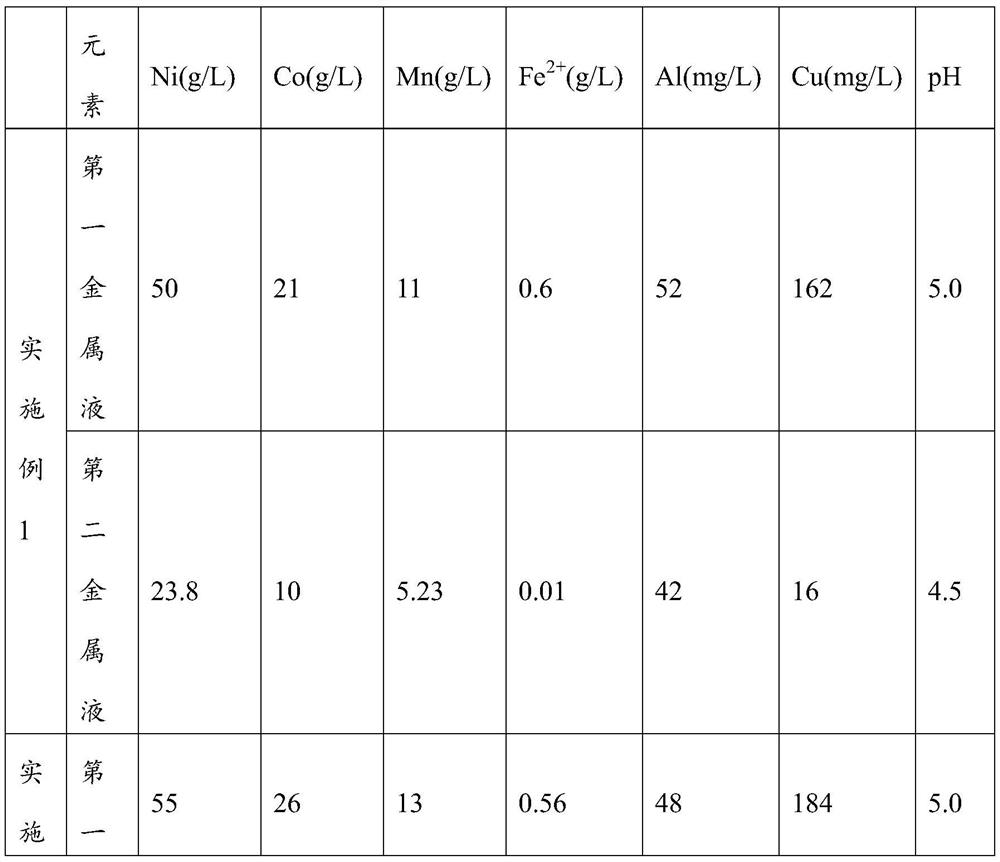
[0040] Take 100g of battery powder, add 106ml of concentrated sulfuric acid with a mass concentration of 70%, mechanically stir slowly for 0.5h, add 300g of water, heat in a water bath at 70°C, and conduct a water immersion reaction for 2h. After the water immersion reaction, the pH of the solution is 5.0. Filter to obtain the first organic Valence metal liquid and first graphite slag;
[0041] The first graphite slag was weighed to be 42g, and the first graphite slag was slurried with 126ml of water, the temperature was raised to 70°C, concentrated sulfuric acid was added dropwise to pH 0.5, and sodium sulfite, which was 1 times the molar amount of the valuable metal in the graphite slag, was added and reacted for 4 hours. Add an appropriate amount of sodium hydroxide dropwise to adjust the pH of the solution to 4.5, react for 1 hour, and filter to obtain the s...
Embodiment 2
[0043] This embodiment reclaims the valuable metals in the waste lithium ion battery, and the specific process is:
[0044] Take 100g of battery powder, add 99ml of concentrated sulfuric acid with a mass concentration of 75%, mechanically stir slowly for 0.5h, add 300g of water, heat in a water bath at 70°C, and conduct a water immersion reaction for 2h. After the water immersion reaction, the pH of the solution is 5.0. Filter to obtain the first Valence metal liquid and first graphite slag;
[0045] Weigh the first graphite slag to be 45g, make slurry with the first graphite slag and 135ml water, raise the temperature to 70°C, add concentrated sulfuric acid dropwise to pH0.5, add sodium sulfite with 1.2 times the molar amount of the valuable metal in the graphite slag, react for 3h, Add an appropriate amount of sodium hydroxide dropwise to adjust the pH of the solution to 4.5, react for 1 hour, and filter to obtain the second valuable metal liquid and the second graphite slag...
Embodiment 3
[0047] This embodiment reclaims the valuable metals in the waste lithium ion battery, and the specific process is:
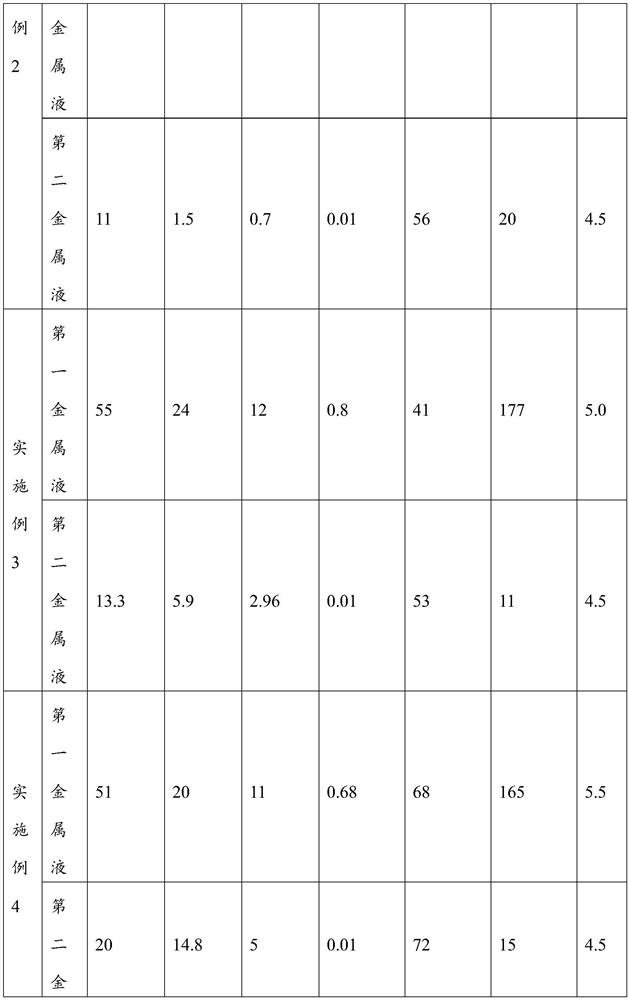
[0048] Take 100g of battery powder, add 93ml of concentrated sulfuric acid with a mass concentration of 80%, mechanically stir slowly for 0.5h, add 300g of water, heat in a water bath at 70°C, and immerse in water for 2h. After the water immersion, the pH of the solution is 5.0, and filter to obtain the first organic Valence metal liquid and first graphite slag;
[0049]Weigh the first graphite slag to be 45g, make slurry with the first graphite slag and 135ml water, raise the temperature to 70°C, add concentrated sulfuric acid dropwise to pH 0.5, add sodium sulfite with 1.3 times the molar amount of the valuable metal in the graphite slag, react for 3h, Add an appropriate amount of sodium hydroxide dropwise to adjust the pH of the solution to 4.5, react for 1 hour, and filter to obtain the second valuable metal liquid and the second graphite slag.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com