Combinations of a hdm2-p53 interaction inhibitor and a bcl2 inhibitor and their use for treating cancer
An HDM201, hdm2-p53 technology, applied in the combination of an HDM2-P53 interaction inhibitor and a BCL2 inhibitor, and the field of use in the treatment of cancer, can solve problems such as lack of efficacy and/or safety, and achieve a favorable therapeutic index, The effect of low morbidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0111] Example 1: HDM201 dosing regimen modeling
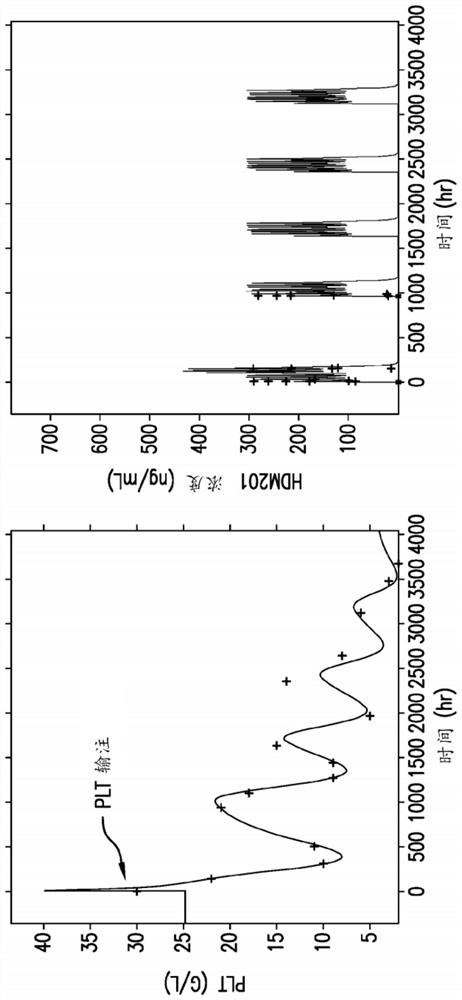
[0112] platelet model
[0113] Based on the population PK / PD data from clinical study CHDM201X2101, an AML patient platelet model was developed that recognizes that the disease affects the regulation of platelet production. The figure below illustrates the described model.
[0114]
[0115] Myeloid Blast Model
[0116] A bone marrow blast PKPD model was developed that recognizes a delayed effect, loss of effect over time that is recapitulated by the resistance component, and recognizes the impact of intensive administration to reduce resistance. The figure below illustrates the described model.
[0117]
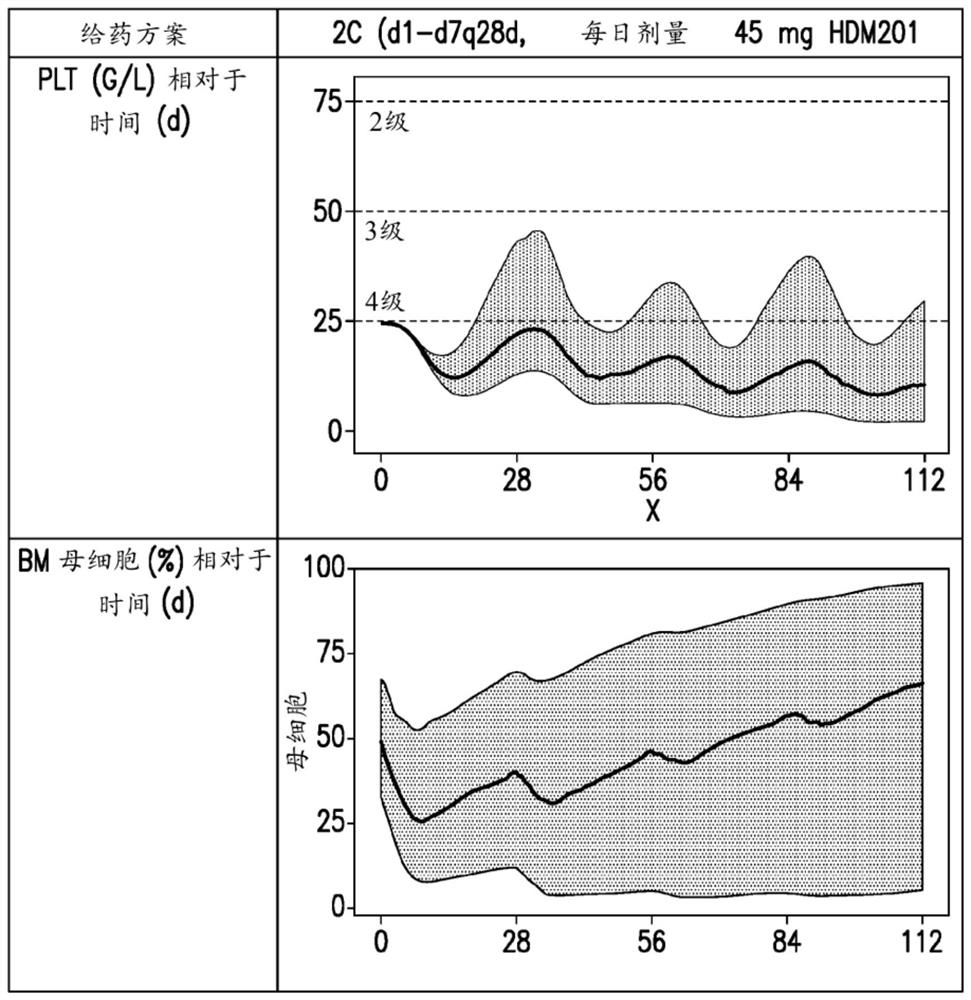
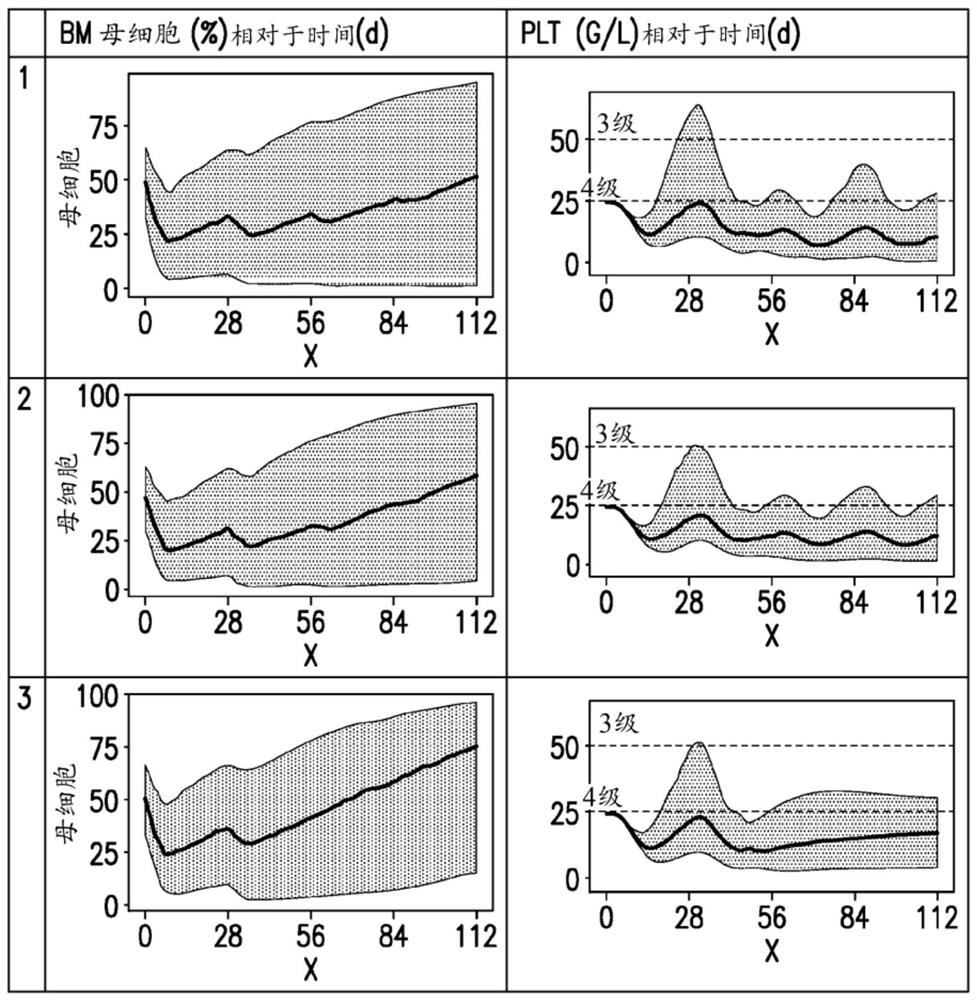
[0118] Derivation of key metrics from simulated platelet and blast curves
[0119] The population PK / PD models of Example 1 and Example 2 were used to simulate time-dependent variation and inter-individual variation in PK, platelet and blast profiles.
[0120] The effect of changes in dosing regimen on thes...
example 2
[0215] Example 2: Preclinical Research
[0216] Example 2: In vivo pharmacology of HDM201 and venetoclax combination
[0217] In multiple AML patient-derived orthotopic models, HDM201 was shown to enhance the in vivo antitumor activity of the selective Bcl-2 inhibitor venetoclax. In mutant IDH1 / FLT3-ITD bearing mice, HDM201 treatment alone exhibited minimal anticancer activity (92% T / C, p>0.05). In contrast, HDM201 in combination with venetoclax induced complete tumor regression (-100% Reg), whereas only partial tumor regression was observed with venetoclax alone (-9% to -52% Reg). See Figures 8 and 9.
[0218] Consistent with observations in peripheral blood, by spleen weight and IDH1 R132H IHC staining of positive leukemic cells also observed depletion of leukemic cells in the spleen. HDM201 as a single agent resulted in modest reductions in spleen size and leukemic density. In contrast, HDM201 in combination with venetoclax resulted in almost complete depletion of le...
example 3
[0220] Example 3: Clinical Research
[0221] Rationale and Design of Dose / Regimen and Duration of Treatment of HDM201 in Combination with Venetoclax
[0222] This is a Phase 1b, multi-arm, open-label study of HDM201 in combination with venetoclax in subjects with AML or high-risk MDS.
[0223] For all subjects, TP53WT status must be characterized by at least the absence of mutations in exons 5, 6, 7, and 8.
[0224] Subjects will receive HDM201 in combination with venetoclax.
[0225] The venetoclax dose will be gradually increased (rising) over a period of 4 to 5 days to reach the target daily dose to be tested (400 or 600 mg), as shown in the diagram below:
[0226] Rise of venetoclax (RU) during cycle 1 (treatment arm 2: HDM201 + venetoclax)
[0227]
[0228] Once subjects have received the planned target daily dose, they will continue with that dose of venetoclax.
[0229] HDM201 doses can be escalated (see Table 3-1 for interim dose levels to be tested). Based...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com