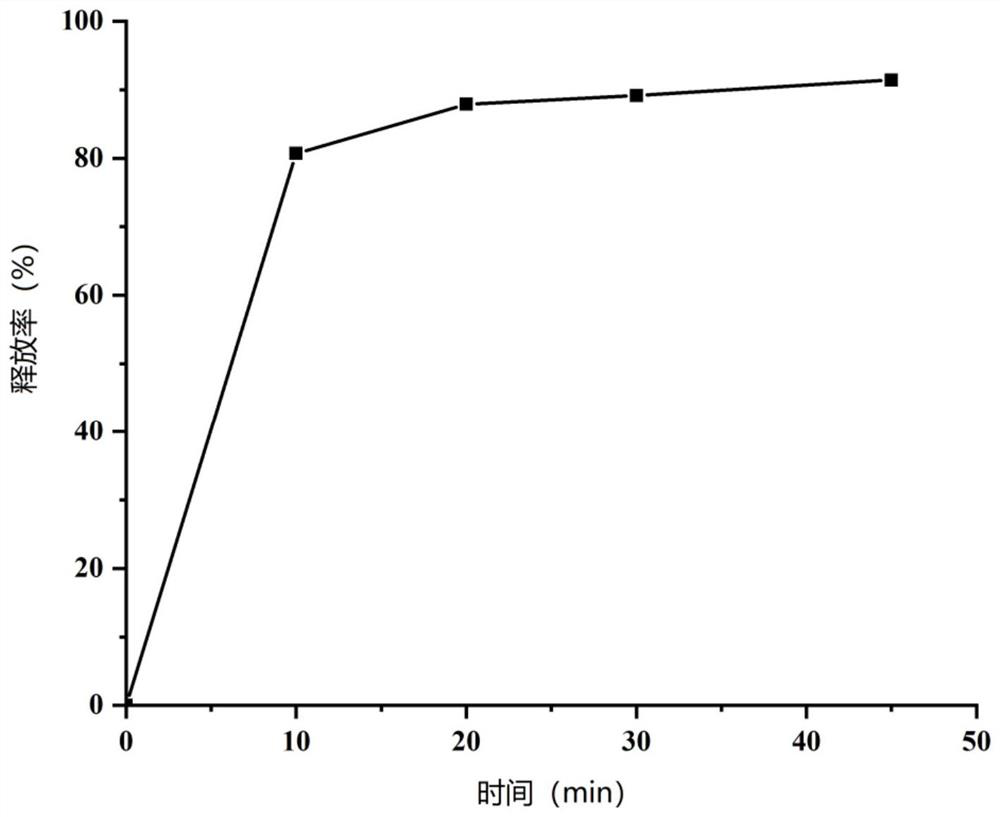
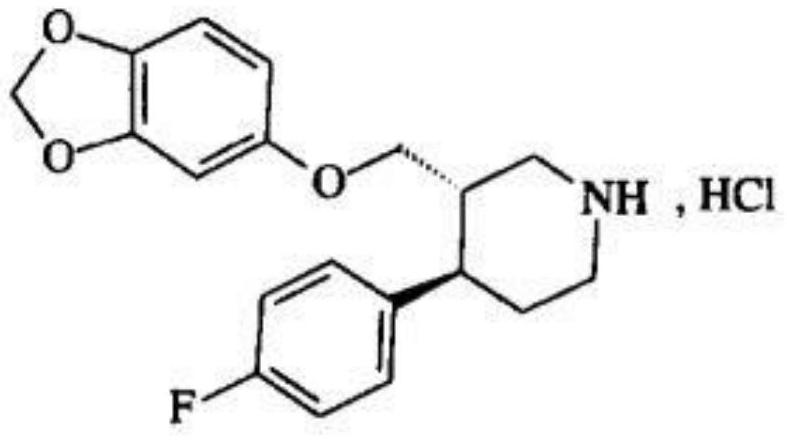
Paroxetine hydrochloride suspension and preparation method thereof
A technology for paroxetine hydrochloride and suspensions, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, and medical preparations containing active ingredients. Limited type, increased plasma concentration of paroxetine, etc., to achieve the effect of increased drug loading, easy swallowing, and improved drug release behavior
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The present embodiment provides a kind of paroxetine hydrochloride suspension (10mL specification), comprises following preparation raw material:
[0055]
[0056]
[0057] Its preparation method is as follows:
[0058] (1) Mix Amberlite IRP69, Amberlite IRP88, and Amberlite IRP64 with water and then mix them with paroxetine hydrochloride, take samples regularly, and measure the concentration of the drug in the solution. When the concentration of the drug in the solution does not change with time, the equilibrium is reached, the unbound drug on the surface of the resin is washed away with deionized water, and dried at 50°C to obtain the drug-loaded resin complex;
[0059] (2) Add the drug-loaded resin complex obtained in step (1) to an aqueous solution of PEG4000 (10%) and PEG3350 (15%), stir at 60°C for 30min, and dry at 50°C to obtain an impregnated drug-loaded resin Complex;
[0060] (3) Mix the impregnated drug-loaded resin complex obtained in step (2) with ...
Embodiment 2
[0062] The present embodiment provides a kind of paroxetine hydrochloride suspension (10mL specification), comprises following preparation raw material:
[0063]
[0064]
[0065] Its preparation method is as follows:
[0066] (1) Mix Amberlite IRP69, Amberlite IRP88, and Amberlite IRP64 with water and place them in the chromatography column, then add the paroxetine hydrochloride solution into the chromatography column for chromatography, and take samples at regular intervals until the drug concentration of the added liquid is equal to that of the effluent. At the same time, the equilibrium is reached, the unbound drug on the surface of the resin is washed away with deionized water, and dried at 45°C to obtain the drug-loaded resin complex;
[0067] (2) Add the drug-loaded resin complex obtained in step (1) to an aqueous solution of PEG4000 (15%) and PEG3350 (10%), stir at 55°C for 40min, and dry at 45°C to obtain an impregnated drug-loaded resin Complex;
[0068] (3) ...
Embodiment 3
[0070] The present embodiment provides a kind of paroxetine hydrochloride suspension (10mL specification), comprises following preparation raw material:
[0071]
[0072]
[0073] Its preparation method is as follows:
[0074] (1) Mix Amberlite IRP69, Amberlite IRP88, and Amberlite IRP64 with water and place them in the chromatography column, then add the paroxetine hydrochloride solution into the chromatography column for chromatography, and take samples at regular intervals until the drug concentration of the added liquid is equal to that of the effluent. At the same time, the equilibrium is reached, the unbound drug on the resin surface is washed away with deionized water, and dried at 60°C to obtain the drug-loaded resin complex;
[0075] (2) Add the drug-loaded resin complex obtained in step (1) to an aqueous solution of PEG4000 (15%) and PEG3350 (10%), stir at 65°C for 20min, and dry at 60°C to obtain an impregnated drug-loaded resin Complex;
[0076] (3) Mix the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com